In the quiet halls of the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague), a revolution in our understanding of matter has quietly taken shape. It’s not a revolution born from loud explosions or particle accelerators, but one that unfolded inside sophisticated molecular models and blink-of-an-eye quantum behavior. What this team of scientists, led by Professor Pavel Jungwirth, has discovered challenges one of the oldest assumptions in physics and chemistry—that materials transform cleanly from one state to another, with a definitive line drawn between them.
The truth, as it turns out, may be far messier—and far more mesmerizing.
Flipping States: Neither Metal Nor Nonmetal, But Both
When a liquid undergoes a transition from a nonmetallic to a metallic state, something strange can happen. According to this new research, published in Nature Communications, there’s a phase in between that doesn’t quite fit into any known category. Instead of smoothly becoming a metal or stubbornly remaining a nonmetal, the system begins to flicker—ultrafast—between both identities.
This phase isn’t like flipping a switch between two rooms. It’s more like standing in a corridor where the walls shimmer, and every few trillionths of a second you’re pulled between two realities.
“It’s not a static condition,” says Prof. Jungwirth. “We’re witnessing a dynamic fluctuation—a rapid, spontaneous jumping back and forth between two fundamentally different electronic states. This wasn’t just unexpected. It was unprecedented.”
And these aren’t slow, leisurely hops. We’re talking about tens of femtoseconds—a femtosecond being one quadrillionth of a second. Blink, and this phase has already switched states billions of times.
How the Electron Broke the Rules
To understand how extraordinary this is, we have to dive into the nature of metals and nonmetals. Metals, in essence, are defined by the presence of free-moving electrons—the so-called conduction band that allows them to carry electricity. Nonmetals, by contrast, lack this electron mobility. But what happens when a material is caught between the two?
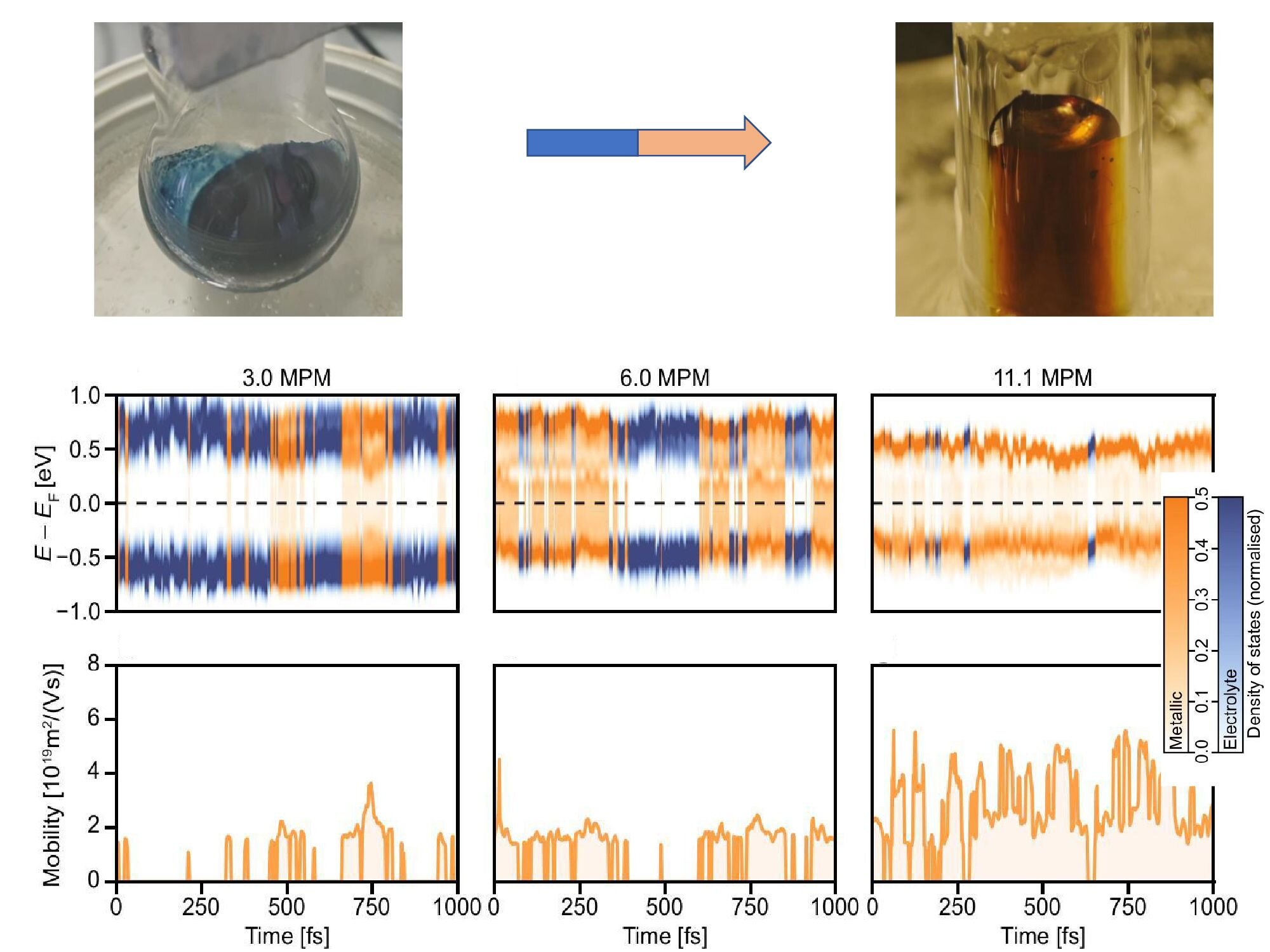
In earlier work that caught international attention, Jungwirth’s team dissolved alkali metals—like lithium or potassium—into liquid ammonia. Initially, the solution was a vivid blue, characteristic of solvated electrons. But as more metal was added, the solution turned golden—marking the moment when it became a liquid metal. Unlike solid metals, this shimmering substance flowed like water, yet conducted electricity like copper wire.
This unusual phase had been described in Science five years ago. It was a major moment in the chemistry of exotic states of matter. But what lay between the blue and the gold remained a mystery—until now.
Simulating the In-Between
Using cutting-edge molecular dynamics simulations, in collaboration with researchers from the University of Oxford, Charles University, and the J. Heyrovský Institute of Physical Chemistry, the team set out to investigate the nature of this transition.
Their simulations didn’t reveal a smooth slide or a sharp snap from one phase to another. Instead, they observed a dynamic instability—a jittering back and forth, where the system didn’t know which identity to settle on. In the simulation’s high-resolution time scale, the liquid didn’t transition once. It oscillated between phases, performing a flickering dance on a femtosecond stage.
This was a new kind of phase behavior—neither a classical two-phase mixture nor a smooth crossover. It was, quite literally, a quantum choreography unfolding at incomprehensible speeds.
Capturing the Uncatchable
Of course, simulations are just the beginning. The challenge now lies in catching this phenomenon in the real world, and that’s no small task.
“This won’t be easy,” admits Marco Vitek, the Ph.D. student and first author on the study. “We’re talking about switching events happening a million times faster than the fastest shutter speed on any ordinary camera. You need laser pulses of incredible brevity—on the order of tens of femtoseconds—to even begin to capture it.”
Luckily, the team is already preparing to do exactly that. Using ultrafast laser systems—some of which they already have access to—they’re designing experiments that could shine light, quite literally, on this ephemeral phase.
Another possible avenue is photoelectron spectroscopy at a synchrotron facility, where high-energy light can eject electrons from the material and allow scientists to probe its electronic structure. By timing these measurements with femtosecond precision, they may be able to catch the liquid in the act of switching.
Why It Matters: The Frontier of Phase Transitions
If this phenomenon is experimentally verified, it will represent the discovery of a new class of phase behavior—one that occurs not in equilibrium, but in a rapid, perpetual oscillation.
Traditionally, phase transitions are considered relatively clean affairs. Water freezes into ice. Metal melts into liquid. Semiconductor materials transition under pressure or doping conditions. But this discovery hints that under the right circumstances—especially in complex fluids—nature might prefer to dither, teetering between states rather than committing to one.
Such behavior could have profound implications in fields ranging from quantum computing to materials science. Devices that rely on fast electronic switching, or that operate under extreme conditions, might be able to harness this flickering state to create new functionalities—like ultrafast switching circuits, sensors with femtosecond sensitivity, or even novel energy storage mechanisms.
Furthermore, this opens up a fundamental question for physicists and chemists alike: Is this flickering transition unique to the systems studied, or is it a universal feature waiting to be discovered in other complex materials?
A New Narrative of the Metallic State
There’s also a philosophical component to this discovery. Metals have always occupied a specific place in our understanding of the material world—conductive, shiny, solid. To find a metal that’s liquid is already strange. To find one that flickers in and out of metallicity is stranger still.
“Metals don’t have to be cold, hard, and silent anymore,” says Jungwirth. “They can be hot, fluid, and—if we’re right—vibrating between identities at speeds that boggle the mind.”
This reframes our picture of what a phase is. Not a static category, but a probabilistic pulse. Something that can exist, not as a point on a line, but as a dynamic region of flux.
The Next Challenge: Proving It
The team is now on a dual mission: to refine their theoretical models and to design experiments capable of capturing the transition in action. This will require collaboration with ultrafast laser labs, time-resolved spectroscopists, and possibly even new types of detection technology.
Still, the atmosphere in the lab is electric—no pun intended.
“There’s something poetic about it,” Vitek muses. “We spend our lives trying to understand how the world works, and then we find that even something as fundamental as a phase might not be stable, but jittery. Like it’s alive.”
Science in Motion
The story of Jungwirth’s team is a testament to the evolving nature of science itself. As our tools grow more precise and our simulations more detailed, we’re beginning to glimpse behaviors in matter that were once hidden behind the veil of time.
The boundary between metal and nonmetal is no longer a sharp threshold—it’s a space of flickering ambiguity, a realm where electrons surge and recede like tides under a quantum moon.
As we continue to explore this liminal state, we may find that nature is less a machine than a dancer—switching steps, shifting rhythms, and always staying just ahead of what we thought we understood.
In the words of Einstein, who spent his life chasing the deeper harmonies of the universe: “The most beautiful thing we can experience is the mysterious.” The flickering phase between metal and nonmetal might just be one of the most beautiful mysteries we’ve yet uncovered.
Reference: Marco Vitek et al, Rapid flipping between electrolyte and metallic states in ammonia solutions of alkali metals, Nature Communications (2025). DOI: 10.1038/s41467-025-59071-z