At the heart of every physical object—every drop of water, grain of salt, living cell, and planetary atmosphere—lies a powerful yet invisible force: chemical bonding. These bonds are not just scientific abstractions; they are the glue that holds matter together. Without them, atoms would drift separately through space, incapable of forming the molecules and compounds that make up the universe, including you and me.
Two of the most fundamental types of chemical bonds—ionic and covalent—are responsible for the vast majority of substances we encounter in daily life. Though they serve a similar purpose—bringing atoms together to form more stable structures—they do so in profoundly different ways. These differences shape the properties of materials, influence biological systems, and determine how substances interact, react, and transform.
To understand what separates ionic bonds from covalent bonds, and why that difference matters, we must begin at the atomic level, where electrons dance between attraction and repulsion, order and chaos.
Atoms: The Will to Bond
Atoms are the basic units of matter, composed of a dense nucleus containing positively charged protons and neutral neutrons, surrounded by a cloud of negatively charged electrons. The number of protons determines the identity of the atom—hydrogen has one, helium two, oxygen eight, and so on. Electrons occupy energy levels around the nucleus in regions called orbitals.
But electrons are not content to simply orbit passively. They crave balance. Atoms seek to achieve a full outer electron shell, also known as the octet rule, based on the stability seen in noble gases. This desire drives atoms to interact with one another—either by transferring electrons or by sharing them.
How atoms fulfill this need defines the nature of the chemical bond they form.
The Birth of an Ionic Bond: A Story of Transfer
An ionic bond is born out of imbalance—one atom has too few electrons, another has too many. When a highly electronegative atom (such as chlorine or oxygen) encounters an atom with low electronegativity (such as sodium or potassium), the stage is set for electron theft. One atom becomes a thief; the other, a willing donor.
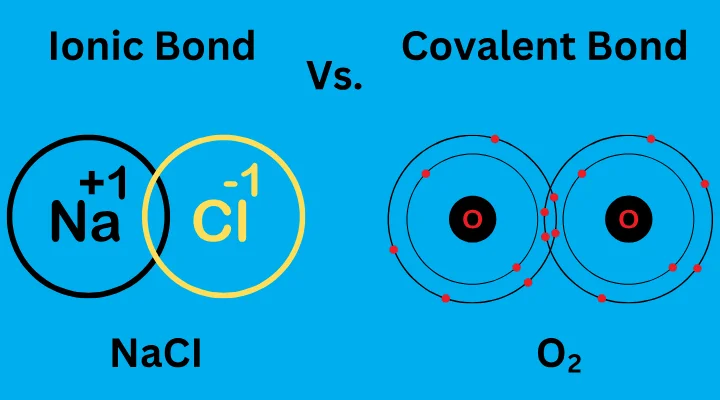
Consider table salt—sodium chloride. Sodium has one electron in its outermost shell, far from its nucleus, held loosely by its low nuclear charge. Chlorine, on the other hand, has seven electrons in its outer shell and a powerful attraction for electrons. When the two meet, sodium transfers its lone outer electron to chlorine. This act transforms sodium into a positively charged ion (Na⁺), and chlorine into a negatively charged ion (Cl⁻).
Opposite charges attract, and the newly formed ions are drawn together, bound not by shared ownership but by raw electrostatic force. This is the essence of the ionic bond: a relationship forged through electron transfer and maintained by electric charge.
The resulting compound—ionic in nature—is typically crystalline, with a repeating lattice structure that maximizes attractions and minimizes repulsions. The regularity of this structure gives ionic compounds their hardness and high melting points.
Covalent Bonds: A Mutual Embrace
Where ionic bonds arise from a difference in electronegativity so extreme that one atom willingly gives up an electron, covalent bonds emerge in a more egalitarian context. Here, atoms come together not as donor and acceptor, but as partners, sharing one or more pairs of electrons to achieve mutual stability.
Take water, the elixir of life. A molecule of water is formed when two hydrogen atoms each share an electron with an oxygen atom. Oxygen needs two electrons to fill its outer shell, and hydrogen needs one. By sharing, each hydrogen atom “feels” like it has two electrons in its outer shell, and oxygen “feels” like it has eight.
In a covalent bond, the electrons are not transferred but shared in a space between the two nuclei. This shared region is known as the bonding orbital, where the electrons are simultaneously attracted to both nuclei. The balance of attractions holds the atoms together.
Covalent bonds can be single (one pair of shared electrons), double (two pairs), or triple (three pairs), depending on how many electrons are shared. The more pairs shared, the stronger and shorter the bond.
These bonds are the foundation of organic chemistry, forming the backbone of carbon-based life. Every protein, DNA strand, enzyme, and cell membrane owes its structure and function to covalent bonding.
Ionic and Covalent: A Spectrum, Not a Binary
Though we often present ionic and covalent bonds as opposites, they are better understood as endpoints on a spectrum of bonding. Most bonds have some degree of both ionic and covalent character, depending on the difference in electronegativity between the atoms involved.
Electronegativity is a measure of how strongly an atom attracts electrons. When two atoms with vastly different electronegativities bond, the result is more ionic in character. When the difference is small—or nonexistent, as in diatomic molecules like O₂ or N₂—the bond is purely covalent.
For example, hydrogen fluoride (HF) is technically a covalent molecule, but the electronegativity difference between hydrogen and fluorine is large enough to give the bond significant ionic character. The electrons spend more time near the fluorine atom, creating a partial negative charge on fluorine and a partial positive charge on hydrogen. This results in a polar covalent bond.
Thus, bonding is not black and white, but shaded in many hues of polarity and electron distribution. Recognizing this spectrum is essential for understanding the subtleties of chemical behavior.
Physical Properties: How Bond Type Shapes the World
The nature of a bond has profound implications for the physical properties of a substance. These differences can be observed in state (solid, liquid, gas), melting point, solubility, conductivity, and more.
Ionic compounds tend to form crystalline solids at room temperature, with high melting and boiling points. The strong electrostatic forces holding the ions in place require a great deal of energy to overcome. In solid form, ionic compounds do not conduct electricity because the ions are locked in place. However, when dissolved in water or melted into a liquid, the ions are free to move, allowing the substance to conduct electricity.
In contrast, covalent compounds vary more widely in physical form. Many are gases or liquids at room temperature, especially those with small molecules like CO₂ or CH₄. Larger covalent compounds, like sugars and proteins, are solids but often lack the rigid crystalline structure of ionic compounds.
Covalent substances typically have lower melting and boiling points compared to ionic compounds because the forces between molecules (intermolecular forces) are weaker than the ionic bonds between ions. Covalent compounds generally do not conduct electricity in any state, as they do not contain free-moving charged particles.
These contrasting properties are not just academic; they determine how substances are used in real-world applications, from building materials to pharmaceuticals.
Solubility and Bonding
One of the classic rules in chemistry—“like dissolves like”—relates directly to the type of bonding in a substance. Ionic compounds are typically soluble in polar solvents like water. The partial charges on water molecules surround and stabilize the positive and negative ions, pulling them into solution.
Covalent compounds show a more varied behavior. Polar covalent molecules like sugar dissolve readily in water, while nonpolar covalent compounds like oil do not. Instead, they dissolve in nonpolar solvents like hexane. The nature of the covalent bond—polar or nonpolar—determines how the molecule interacts with its environment.
This principle governs everything from nutrient absorption in the body to industrial chemical processes.
Biological Relevance of Bonding Types
In biological systems, both ionic and covalent bonds play essential roles, but in very different ways.
Covalent bonds form the backbone of biomolecules. DNA, proteins, carbohydrates, and lipids are all built from atoms linked by covalent bonds. These bonds provide the stability and specificity needed to maintain complex structures and carry out precise functions.
However, ionic interactions are equally crucial. Within the watery environment of cells, ions like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) are constantly moving across membranes, generating electrical signals that control nerve impulses, muscle contractions, and heartbeats. Ion channels, pumps, and gradients are vital components of cellular life.
Furthermore, ionic and hydrogen bonds (a type of weak interaction related to polarity) help stabilize the three-dimensional structure of proteins and DNA. Enzyme activity, receptor binding, and molecular recognition often depend on these temporary, reversible interactions.
The interplay of ionic and covalent bonding in biological systems is a symphony of stability and flexibility, enabling life to be both robust and responsive.
Bond Strength and Reactivity
Another key distinction between ionic and covalent bonds lies in their reactivity. Ionic bonds, while strong in the solid state, can be easily disrupted in polar solvents. This makes ionic compounds good candidates for reactions in aqueous environments. In solution, the separated ions are free to interact, combine, or be replaced in chemical reactions.
Covalent bonds, on the other hand, require specific conditions to break or form. Chemical reactions involving covalent molecules often involve catalysts, heat, or enzymes. Once formed, covalent bonds are typically more stable and less reactive than ionic interactions.
This difference explains why salts like NaCl dissolve and dissociate easily in water, while organic molecules like glucose maintain their integrity. It also underpins much of synthetic chemistry, pharmacology, and metabolic pathways.
Beyond Simple Models: Coordinate and Metallic Bonds
While the dichotomy of ionic versus covalent is foundational, the world of chemical bonding contains other fascinating varieties that blend or extend these concepts.
Coordinate covalent bonds, for instance, occur when both electrons in a shared pair come from the same atom. This type of bonding is common in transition metal complexes and plays a major role in biological systems like hemoglobin, where iron binds oxygen through coordinate bonds.
Metallic bonding, another distinct type, involves a sea of delocalized electrons shared among a lattice of metal cations. This unique structure gives metals their characteristic properties: conductivity, malleability, and luster. Though not ionic or covalent in the traditional sense, metallic bonding is yet another way nature binds atoms together.
Understanding these additional bonding types enriches our view of chemical structures and expands our ability to design new materials.
Teaching and Learning the Difference
The distinction between ionic and covalent bonds is often introduced early in chemistry education. Students are taught to recognize ionic compounds by the presence of a metal and nonmetal, and covalent compounds by the presence of two nonmetals. Electronegativity differences are used as a guide to predict bond type.
However, real-world chemistry is rarely so tidy. Many compounds exhibit characteristics of both types of bonding. Polyatomic ions, for example, contain covalent bonds within a charged group that participates in ionic bonding with other ions. Transition metal compounds can defy classification entirely, displaying complex bonding behavior that challenges traditional rules.
Effective teaching must go beyond memorization, helping students develop an intuition for how atoms interact, why they do so, and what that means for the behavior of matter.
Ionic and Covalent Bonds in the Cosmos
Bonding is not just a laboratory phenomenon; it governs the chemistry of stars, planets, and interstellar space. In the high temperatures of stars, hydrogen atoms fuse into helium through nuclear processes, not chemical bonding. But in the cooler outskirts of the universe, molecules form through both ionic and covalent bonds.
The icy moons of Jupiter and Saturn, the methane lakes of Titan, and the carbon-rich asteroids of the Kuiper Belt are all testaments to the diverse chemistry shaped by bonding.
Even in the harsh vacuum of space, covalent molecules like water, carbon monoxide, and ammonia have been detected. Their formation tells a story of how complexity arises from simplicity, of how molecules can survive the vastness of space and perhaps seed the raw materials for life.
A Final Reflection: Bonding as the Language of Chemistry
At its core, chemistry is about transformation—how substances change, react, and recombine to form new substances. Bonding is the language through which these transformations occur. Understanding the difference between ionic and covalent bonds is like learning the grammar of that language.
Ionic bonds represent clarity and structure, forged through strong opposites and governed by rules of charge and symmetry. Covalent bonds represent nuance and subtlety, formed through sharing, shaped by geometry, and influenced by polarity.
Together, they account for the richness of chemistry, the diversity of matter, and the marvel of life. The next time you taste a pinch of salt, sip a glass of water, or gaze at the night sky, remember that everything you see, touch, and feel is held together by the silent force of chemical bonding.
It is the quiet architect of reality.