Take a moment to look around. The chair you sit on, the device you’re holding, the clothes you’re wearing, and even the cells in your body—everything is held together by polymers. These remarkable substances are among the most versatile and important materials on Earth. They can be soft or hard, elastic or brittle, natural or synthetic. They form the foundation of our modern world, and yet most people rarely give them a second thought.
Polymers are the molecules behind plastics, rubbers, textiles, proteins, and even your genetic code. They’re not a single substance but a vast category of compounds that share one crucial trait: they’re made up of repeating units. This deceptively simple structure allows for astonishing complexity and function.
Let’s unravel the mystery of polymers—how they work, how they’re made, and how they stretch from grocery bags to the double helix of DNA. In doing so, we’ll uncover a hidden architecture that weaves together life, industry, and innovation.
What Are Polymers? The Basics Behind the Buzzword
At its most fundamental level, a polymer is a large molecule composed of many smaller, repeating units called monomers. The word “polymer” comes from the Greek “poly” (many) and “meros” (parts), a fitting name for something that’s essentially a molecular chain.
Imagine a necklace where each bead is a monomer. When strung together in long chains, those beads form a polymer. But unlike a necklace, the polymer chain isn’t just decorative—it has structure, strength, and function. Depending on the monomers involved and how they’re arranged, a polymer can behave like stretchy rubber, rigid plastic, or silky thread.
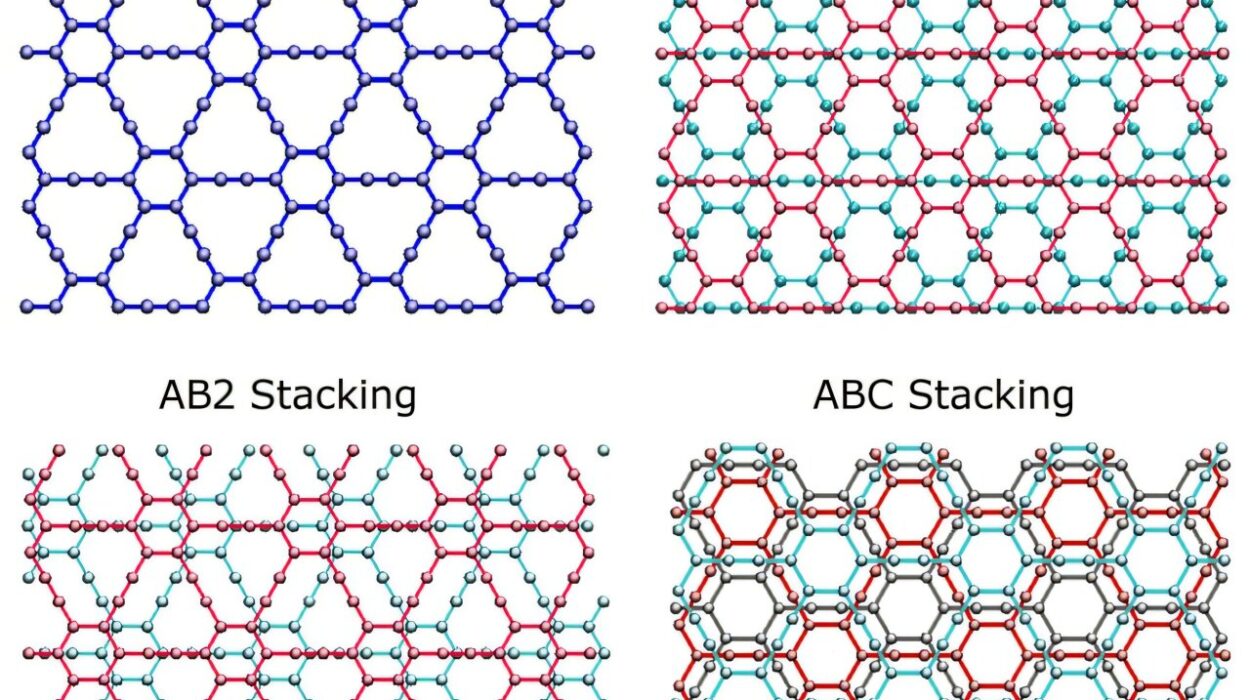
Monomers are usually simple organic molecules, often carbon-based. When they bond chemically through processes like polymerization, they create chains that can reach lengths of thousands or even millions of units. Some polymers are linear, like a straight strand of spaghetti, while others are branched or even crosslinked, like a net or a mesh.
This structural variety gives rise to polymers’ incredible range of properties. A change in the type of monomer, the length of the chain, or the arrangement of the units can radically alter how a polymer behaves.
Polymerization: Building Big Molecules from Small Beginnings
So how do tiny monomers transform into massive polymer chains? The answer lies in a process known as polymerization. There are two main types: addition polymerization and condensation polymerization.
In addition polymerization, monomers add to one another without forming any by-products. This usually involves monomers with double bonds—think of ethylene, which is the base for polyethylene, one of the most common plastics in the world. When triggered by heat, pressure, or a catalyst, the double bonds open up and link together, forming long chains.
In condensation polymerization, monomers join together while releasing a small molecule as a by-product, often water or methanol. A classic example is the formation of nylon from diamine and dicarboxylic acid monomers, where each new bond formed spits out a water molecule.
Some polymers are formed through natural processes—DNA, proteins, cellulose—while others are manufactured in reactors and chemical plants. Regardless of origin, the underlying principle is the same: start small, build big, and change the world.
Natural Polymers: Nature’s Original Engineers
Long before humans began crafting synthetic polymers, nature was already fluent in polymer chemistry. The cells of every living organism are packed with natural polymers, the most important of which are proteins, nucleic acids, and polysaccharides.
Proteins are made from amino acids linked in a specific sequence. The order of these monomers determines the protein’s function, whether it becomes an enzyme catalyzing life-sustaining reactions or the structural keratin in your hair.
DNA, perhaps the most famous polymer of all, is composed of nucleotide monomers. Its iconic double helix structure is built from just four types of nucleotides—adenine, thymine, cytosine, and guanine. The sequence of these units encodes the blueprint for all living things. It’s a polymer with purpose, memory, and logic.
Cellulose, a polymer of glucose, forms the rigid walls of plant cells and is the most abundant organic polymer on Earth. Chitin, another glucose polymer, provides the exoskeleton for insects and crustaceans. Starch, also made of glucose, stores energy in plant cells.
These natural polymers are not just chemically complex—they are exquisitely functional. They fold, twist, and interact with each other to create the machinery of life itself.
Synthetic Polymers: Humanity’s Invention of the Future
The industrial revolution of polymers began in the early 20th century with the invention of synthetic plastics. Bakelite, invented in 1907, was the first fully synthetic polymer, a heat-resistant material used in everything from radios to kitchenware.
From there, a flood of synthetic polymers entered the market: polyethylene, polypropylene, polystyrene, polyvinyl chloride (PVC), nylon, Teflon, and polyester, to name just a few. These materials were cheap, versatile, and strong—perfect for the demands of a rapidly modernizing world.
Polyethylene, the polymer behind plastic bags and bottles, is lightweight and chemically resistant. Nylon, developed in the 1930s, revolutionized the textile industry with its strength and elasticity. Teflon, famous for non-stick cookware, has one of the lowest coefficients of friction of any solid.
Synthetic polymers can be tailored to exhibit almost any property: conductivity, elasticity, transparency, rigidity. Scientists manipulate molecular weight, branching, cross-linking, and the inclusion of additives to achieve desired results.
Today, synthetic polymers are used in everything from medical devices and space exploration to clothing, construction, and electronics. They have changed the face of modern life—and not always for the better.
The Problem of Plastic: A Polymer Crisis
As useful as synthetic polymers are, they come with a dark side. Many of them, especially plastics, are incredibly resistant to degradation. What makes them durable in your kitchen or car also makes them persistent in the environment.
Each year, over 300 million tons of plastic are produced globally, and a significant portion ends up as waste. Oceans are littered with plastic debris, and microplastics—tiny fragments of polymers—have been found in fish, drinking water, and even human blood.
The longevity of these materials is due to their strong carbon-carbon backbones and the lack of enzymes in nature that can break them down. Unlike cellulose or proteins, which degrade naturally, synthetic polymers linger for centuries.
Efforts are underway to develop biodegradable plastics and improved recycling techniques. Some research focuses on engineering enzymes or microbes that can digest synthetic polymers. But the challenge remains enormous.
Polymers gave us an industrial miracle, but managing their lifecycle is one of the greatest environmental challenges of our time.
Cross-Linking: Turning Spaghetti into Structures
Polymers can be thought of as long, flexible strands. But in many applications, flexibility isn’t enough. That’s where cross-linking comes into play.
Cross-linking involves chemically bonding different polymer chains together, forming a three-dimensional network. Imagine the difference between cooked spaghetti (uncross-linked) and a woven basket (cross-linked). The former is floppy and weak; the latter is structured and strong.
Vulcanized rubber is a famous example. Natural rubber is sticky and deforms easily. By adding sulfur and heating it—a process called vulcanization—cross-links form between the polymer chains, dramatically improving elasticity and durability.
In hydrogels, used in medical applications like contact lenses or wound dressings, cross-linking helps trap water inside a polymer matrix while maintaining structural integrity.
Cross-linking allows polymers to move beyond the limitations of their linear forms. It turns flowy substances into robust, resilient materials used in tires, coatings, foams, and adhesives.
Thermoplastics vs. Thermosets: The Heat Test
Polymers fall into two broad categories when it comes to heat behavior: thermoplastics and thermosets.
Thermoplastics can be melted, reshaped, and cooled repeatedly without changing their chemical structure. They are like wax—you can reheat and remold them as needed. This makes thermoplastics ideal for manufacturing and recycling. Polyethylene, polypropylene, and polycarbonate are classic examples.
Thermosets, by contrast, undergo a chemical transformation when heated. Once set, they cannot be remelted. Their cross-linked structures make them hard, durable, and heat-resistant. Epoxy resins, phenolic resins, and melamine are all thermosets.
This distinction has practical implications. Thermoplastics dominate consumer goods and packaging, while thermosets are used in applications that require permanence and strength—like circuit boards, aerospace components, and car parts.
Smart Polymers: Responsive Materials of the Future
The next generation of polymers is not just strong or flexible—they’re smart. Smart polymers are materials that respond to external stimuli like temperature, pH, light, or electric fields. They change their properties in real time, adapting to their environment.
One example is shape-memory polymers, which can be deformed and then return to their original shape upon heating. These are being used in medical stents, self-healing materials, and soft robotics.
Stimuli-responsive hydrogels can swell or shrink based on pH changes, making them useful in drug delivery systems that release medication only under specific conditions.
Conductive polymers can carry electrical current, opening possibilities for flexible electronics, biosensors, and wearable tech.
The age of smart polymers is just beginning. These materials blur the line between chemistry and computation, between matter and machine.
Polymers in Biology: From Collagen to Chromosomes
In biology, polymers are not optional—they are fundamental. Life, as we know it, is polymer-based.
DNA and RNA store genetic information and pass it from generation to generation. Proteins serve as enzymes, hormones, structural components, and immune defenders. Polysaccharides like glycogen and starch store energy.
Even the extracellular matrix—the structural framework that holds cells together—is made of polymeric proteins like collagen and elastin.
Inside cells, the cytoskeleton is a dynamic polymer network that gives shape to the cell and enables motion. Microtubules and actin filaments grow and shrink as needed, demonstrating the kind of adaptability synthetic polymers still strive to achieve.
Studying biological polymers not only deepens our understanding of life but inspires new kinds of materials—biomimetic polymers that replicate nature’s elegant designs.
The Endless Possibilities of Polymer Science
From packaging to prosthetics, from circuit boards to cartilage, polymers are the molecules that connect chemistry to the real world. They are the versatile foundation upon which modern society is built—and they hold the keys to many of our future technologies.
The challenges are real: plastic pollution, recycling inefficiencies, and limited biodegradability. But the opportunities are just as vast. With advances in green chemistry, biodegradable materials, and smart responsive polymers, the next century of polymer science may be as revolutionary as the last.
We are only beginning to scratch the surface of what polymers can do. Whether we’re engineering synthetic muscles, 3D-printing organs, or designing self-repairing materials, polymers will be there—quietly, invisibly, making it all possible.
They are the long chains that tie molecules to miracles, chemistry to civilization, and atoms to art.