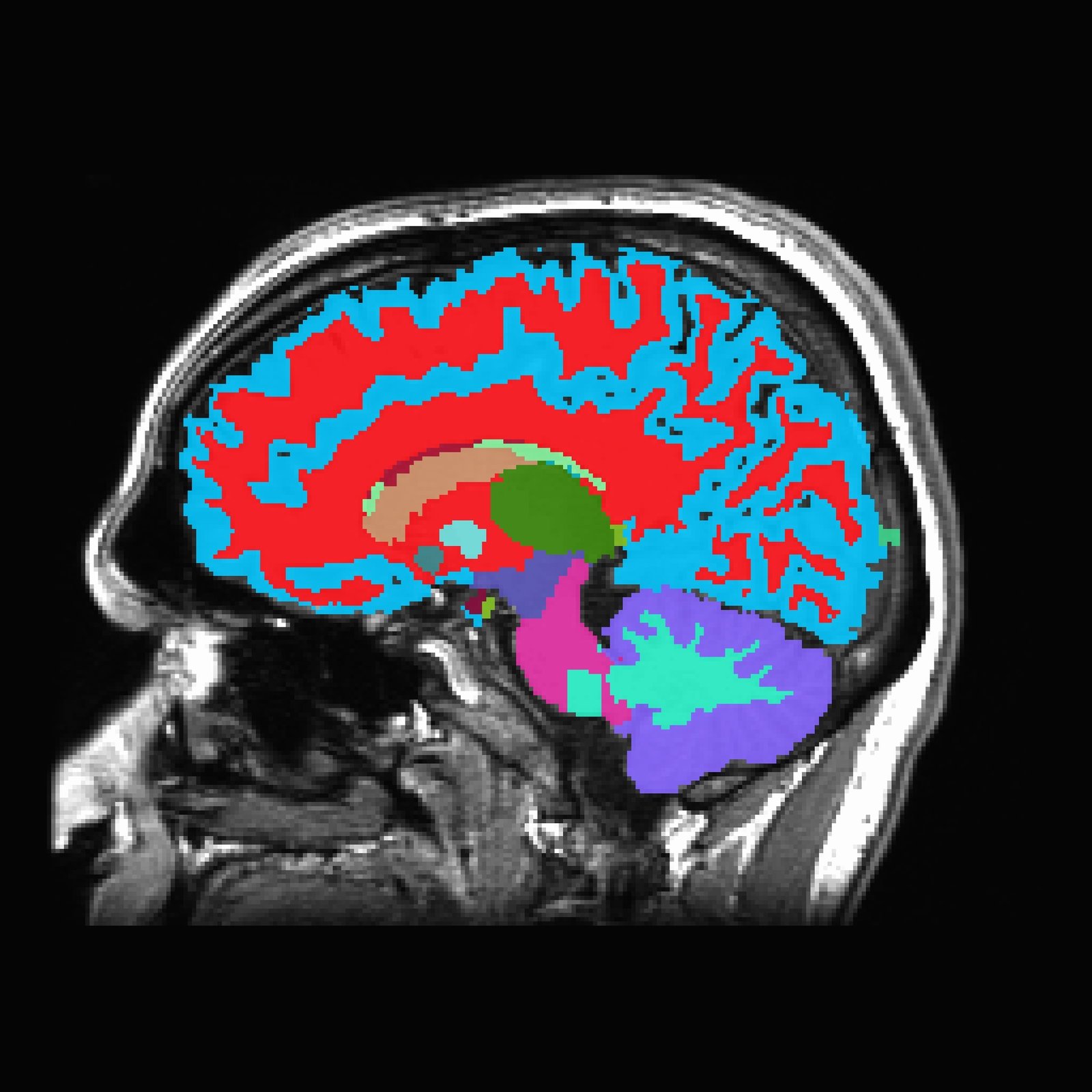
For decades, the human brain was thought to lack the kind of waste-disposal system found in other parts of the body. But now, Columbia University researchers are revealing that not only does the brain have its own version of internal housekeeping—it may be the key to battling one of the most devastating neurodegenerative diseases of our time.
Their latest discovery, published in Neuron, shines a spotlight on a self-cleaning system nestled deep within the brain, known as the glymphatic system. Often described as the brain’s “dishwasher,” this vital cleaning mechanism has captured the imagination of scientists since its discovery a little over ten years ago. Now, it may be the missing piece in the fight against Alzheimer’s disease.
The Brain’s Secret Plumbing System
The glymphatic system—so named because it depends on glial cells and mimics the lymphatic system of the rest of the body—is responsible for sweeping away toxic waste that builds up in the brain. Think of it as a silent janitorial service, active mostly during sleep, rinsing out dangerous proteins like amyloid-beta and tau—two key culprits in Alzheimer’s disease.
In healthy brains, this system operates like a well-oiled machine. Cerebrospinal fluid flows along specialized pathways, guided by star-shaped cells called astrocytes, carrying away metabolic byproducts and potentially harmful debris. But in brains affected by Alzheimer’s, this system becomes sluggish and disorganized. Waste begins to accumulate, forming the sticky plaques and tangles that gradually choke off memory, cognition, and personality.
A Clue from Cancer Drugs
Dr. Guang Yang, associate professor of anesthesiological sciences at Columbia University’s Vagelos College of Physicians and Surgeons, and her team set out to explore why the glymphatic system breaks down in Alzheimer’s—and whether it could be restored.
What they discovered was both startling and promising: a protein known as PERK, commonly involved in cellular stress responses and already being targeted in cancer treatments, plays a pivotal role in misdirecting the very components that make the brain’s cleaning cycle function.
“PERK is like a traffic director gone rogue,” Yang explains. “Instead of guiding the essential cleaning channels to their proper stations, it’s sending them all over the place. And that disorder compromises the brain’s ability to clean itself.”
These channels—known as AQP4 water channels—are supposed to sit neatly at the endfeet of astrocytes, where they manage the movement of fluid that powers glymphatic clearance. But in Alzheimer’s, those channels are scattered around the cell, like buckets in the wrong rooms during a flood. The result? A cleanup system that doesn’t work.
Watching the Brain Clean Itself—Or Not
To get a closer look, the researchers performed a cutting-edge experiment. They injected fluorescent tracer molecules into the cerebrospinal fluid (CSF) of mice and used high-resolution, time-lapse microscopy to track how the molecules moved through the brain’s glymphatic system.
In healthy mice, the tracer flowed smoothly, indicating efficient clearance. But in mice modeled to mimic Alzheimer’s disease, the flow was erratic and diminished. The cleaning system wasn’t just slow—it was broken.
However, when the team inhibited PERK—either by genetically silencing the gene or by using a PERK-blocking drug already in clinical trials for cancer—something remarkable happened. The AQP4 channels migrated back to their rightful positions, glymphatic flow improved, toxic proteins were cleared more effectively, and, most strikingly, the mice performed better on cognitive tasks.
“It was like fixing the plumbing,” Yang says. “Once we got those channels back in place, the brain could start cleaning up again.”
Why This Matters: A New Hope for Alzheimer’s?
Alzheimer’s has long defied treatment. Despite billions of dollars invested in research, the disease remains incurable, affecting over 6 million Americans and tens of millions worldwide. Most existing drugs merely slow down symptoms without addressing the root causes.
Yang’s study doesn’t offer a magic cure, but it opens a promising new door: what if instead of focusing only on the buildup of amyloid and tau, we focused on why the brain is failing to clean these proteins up in the first place?
That’s where the glymphatic system comes in.
“This study gives us a completely different angle to approach Alzheimer’s,” says Dr. Maiken Nedergaard, a neuroscientist who co-discovered the glymphatic system but was not involved in the study. “Instead of just attacking the plaque, we may be able to empower the brain to clean itself. That’s a game changer.”
From Mice to Medicine Cabinets?
Of course, what works in mice doesn’t always translate to humans. But the fact that PERK inhibitors are already being tested in cancer clinical trials is a huge advantage. These drugs have been shown to be safe in early-stage trials, meaning their journey toward Alzheimer’s treatment could be faster than a brand-new compound.
Still, Yang urges caution. “This is just the starting point,” she says. “We know PERK is important in regulating the placement of these AQP4 channels, but we don’t yet fully understand how or why. There’s a complex molecular dance going on, and we’re only just beginning to learn the steps.”
Sleep: The Unsung Hero of Brain Health?
Interestingly, the glymphatic system doesn’t operate at full throttle around the clock. It’s most active during deep sleep, particularly during the slow-wave phase of non-REM sleep. This may explain why sleep disorders are so closely linked to Alzheimer’s and why poor sleep often precedes memory problems by years.
Yang and her team are now investigating whether PERK activity is influenced by sleep, and whether sleep deprivation could trigger or worsen glymphatic dysfunction. It’s a tantalizing hypothesis: if poor sleep impairs the brain’s ability to clean itself, it might set the stage for neurodegeneration years down the line.
“There’s a very real possibility,” Yang notes, “that sleep quality could directly affect how well your brain detoxes itself. And if we can understand that mechanism, we might find even more ways to protect the brain—through lifestyle, through medicine, or ideally both.”
The Glymphatic System: A Common Denominator?
Alzheimer’s may be the most famous neurodegenerative disease, but it’s far from the only one. Parkinson’s, Huntington’s, and frontotemporal dementia also involve the buildup of misfolded proteins. If glymphatic dysfunction is involved in all of them, then targeting this system could have broad therapeutic potential.
“Our findings suggest that glymphatic impairment may be a common pathway in many neurological diseases,” Yang says. “That’s a huge insight. It means we’re not just chasing symptoms—we might be getting closer to the source.”
A New Era in Brain Health?
For generations, the brain has been viewed as a kind of biological fortress—sealed off from the immune system, lacking a traditional lymphatic network, and largely isolated. But the discovery of the glymphatic system turned that view on its head. And now, with this new research, scientists may finally be figuring out how to fix the brain’s plumbing when it breaks down.
The implications extend far beyond Alzheimer’s. Better sleep, smarter drug development, and even early diagnostics could all emerge from this line of research. And while it’s still early days, the momentum is real.
“We used to think of Alzheimer’s as an unstoppable disease,” Yang reflects. “But now we’re starting to see glimmers of hope. If we can empower the brain to heal and cleanse itself, we might not just slow the disease—we might stop it before it starts.”
Reference: Kai Chen et al, Selective removal of astrocytic PERK protects against glymphatic impairment and decreases toxic aggregation of β-amyloid and tau, Neuron (2025). DOI: 10.1016/j.neuron.2025.04.027