Imagine a world where you no longer need to visit the clinic each fall for your annual flu shot. That future may be closer than ever, thanks to a revolutionary development from the University of Nebraska–Lincoln. Virologist Dr. Eric Weaver and his team have unveiled a vaccine strategy with the potential to end the cycle of yearly flu immunization. Their approach, published in Nature Communications, could pave the way for a universal flu vaccine—one that grants protection across strains, species, and even decades.
The new vaccine, built using a computational tool called Epigraph, doesn’t just protect against the common H1N1 “swine flu” virus. It also shows promise in safeguarding against a broad array of influenza A viruses in humans, birds, and pigs. According to Weaver, this scientific leap could “cut off the evolutionary arsenal” of the flu virus, rendering annual shots obsolete.
Why Influenza Is So Hard to Beat
Influenza A virus is notoriously slippery. Infecting up to 15% of the global population each year, it causes thousands of deaths and considerable economic strain. The main problem lies in the virus’s talent for rapid evolution. Flu viruses mutate quickly and possess enormous genetic diversity, especially in their hemagglutinin proteins—the molecular spikes that help them infiltrate human cells.
To make matters worse, flu viruses don’t just affect humans. They also infect birds, pigs, horses, and even dogs. Pigs, in particular, are what scientists call “mixing vessels”—animals capable of being infected by both avian and human strains. This allows for the dangerous reshuffling of viral genes, producing new variants that can leap back into the human population.
This phenomenon, called zoonotic transmission, has been the root cause of numerous global pandemics. The 1918 Spanish Flu, 1957 Asian Flu, 1968 Hong Kong Flu, and the more recent 2009 swine flu pandemic all originated in animals before making their deadly jump into human hosts.
Enter Epigraph: A Smarter Way to Build Vaccines
The Epigraph software behind Weaver’s vaccine is a game changer. By analyzing over 6,000 influenza A virus strains from 1930 to 2021, the software identified the most common “epitopes”—the viral fragments that trigger the human immune system. These epitopes are the secret sauce in teaching the body to defend itself, prompting the production of neutralizing antibodies and virus-killing T-cells.
The resulting vaccine isn’t just based on a snapshot of one current strain, like today’s seasonal flu vaccines. Instead, it’s a cocktail that mirrors the shared features of flu viruses across time and species. The Epigraph strategy aims to future-proof immunity by covering the virus’s most essential and unchanging features.
According to Weaver, this computational approach offers a stark contrast to conventional vaccine design. “What I see on the horizon is a third wave,” he said, “where we go from good vaccines to universal lifelong vaccines.”
Putting the Vaccine to the Test: Swine Trials
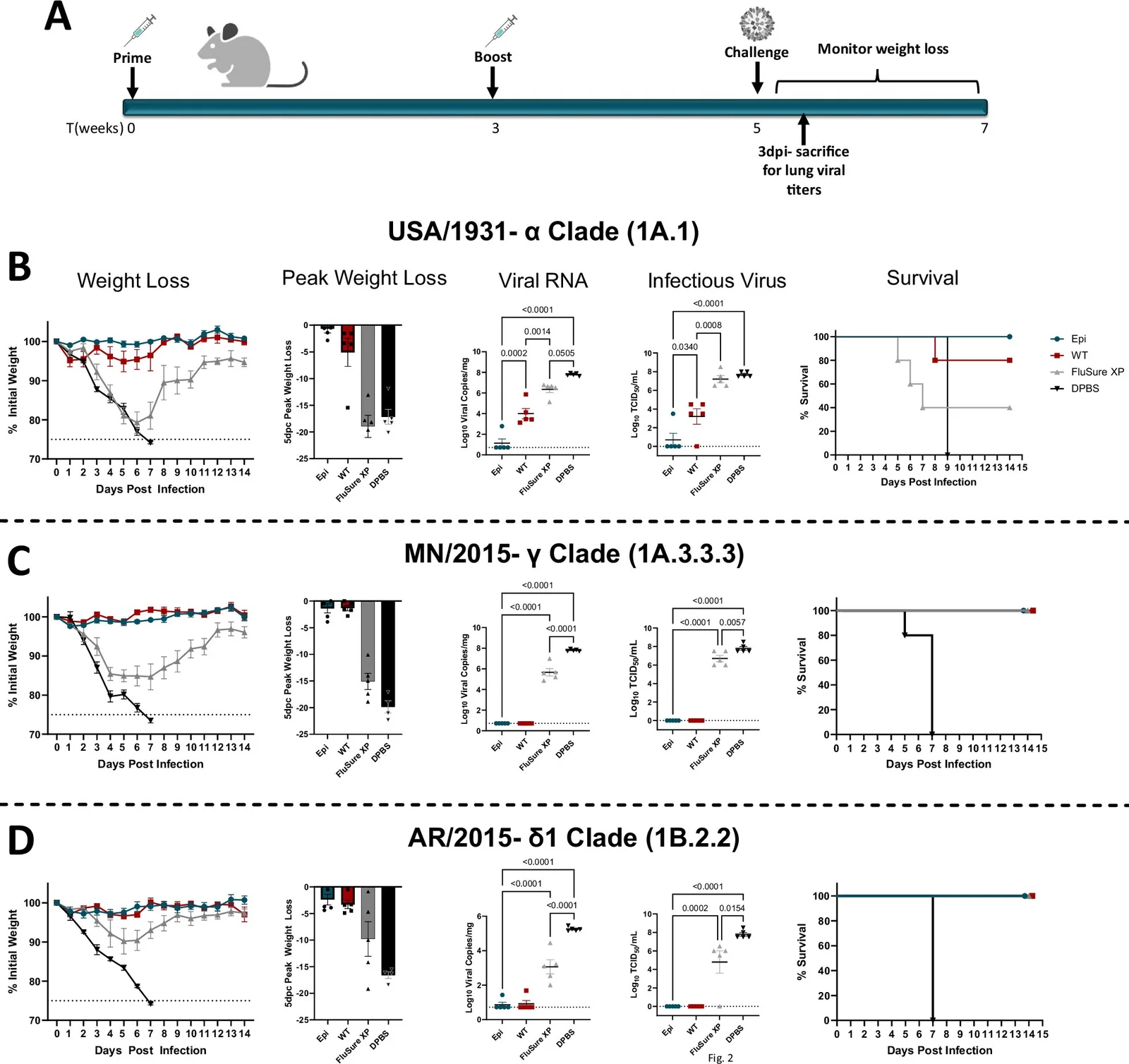
To evaluate the vaccine’s real-world efficacy, Weaver’s lab conducted a rigorous study in swine—an ideal test subject due to their similarity to humans in flu susceptibility. In the trial, four groups of pigs were vaccinated with one of the following: the Epigraph vaccine, a commercial flu vaccine used in the pork industry, a “wild type” vaccine based on natural virus strains, or a placebo.
The results were dramatic.
- Epigraph-vaccinated pigs developed strong antibody responses to all 12 tested strains, including the 2009 pandemic H1N1 variant, several bird flu strains, and human flu viruses from the past 20 years.
- Wild type-vaccinated pigs showed immunity to eight of 12 strains.
- Commercially vaccinated pigs reached immunity thresholds for only six of the 12 strains, and their antibody levels were three to five times lower than those seen in Epigraph-vaccinated pigs.
Even more encouraging was the T-cell data. T-cells, which destroy infected cells, were significantly more active in pigs that received the Epigraph vaccine, suggesting that the immune response was not only broad but also robust.
Immunity That Could Last a Decade or More
One of the most tantalizing findings came from long-term immunity modeling. Over a six-month longitudinal study, the pigs’ immune responses remained stable. Post-experimental regression analysis hinted that immunity induced by the vaccine could persist for a decade or more—a quantum leap over the current seasonal vaccines, which offer only a few months of protection.
For context, today’s flu vaccines must be reformulated and administered every year due to the virus’s shape-shifting nature. Yet even then, vaccine efficacy can vary dramatically from year to year, sometimes dropping as low as 10-20%. The Epigraph approach, if validated in human trials, could transform this frustrating cycle into a thing of the past.
The High-Stakes Battle Against Flu Pandemics
The implications of this research go far beyond convenience. Universal flu protection could dramatically reduce the threat of future pandemics. If pigs are immunized against a wide array of influenza strains, they can no longer act as viral incubators, interrupting the chain of zoonotic transmission.
“If we can prevent influenza in swine,” Weaver emphasized, “we can also prevent zoonotic jumps from avians to swine to humans, or from swine directly to humans.”
In essence, vaccinating animals becomes a strategy to protect people, a concept called “One Health” that recognizes the interconnection between human, animal, and environmental health.
Looking Ahead: From Pigs to People
Weaver’s team is now moving forward with the next stage: testing a combined vaccine that targets both H1 and H3 subtypes of influenza. Given that H1 subtypes are not only more common in pigs but also vastly more genetically diverse than H3, proving efficacy against both would represent a monumental step toward true universality.
Human trials are the ultimate goal, but transitioning from animal models to widespread human use requires substantial funding, partnerships, and regulatory approval. Weaver hopes to collaborate with a biotechnology company to make this vision a reality.
Even in its early stages, however, the Epigraph vaccine has the scientific community buzzing. Not only does it outperform commercial vaccines in pigs, but it also provides data-driven proof that computational vaccine design can succeed where traditional methods have struggled.
A Universal Future for Vaccination?
The development of a universal flu vaccine has been a holy grail of immunology for decades. The constantly evolving nature of influenza has thwarted every attempt—until now. Weaver’s approach, powered by bioinformatics and a deep understanding of viral evolution, may represent the key to unlocking lifelong immunity.
And this innovation may be just the beginning. The computational design principles used for Epigraph can be adapted to other viruses as well, potentially leading to better vaccines for HIV, coronaviruses, and emerging pathogens.
“Our ability to understand how viruses evolve has increased exponentially in the past 20 years,” Weaver said. “We’re on the cusp of designing vaccines not just to chase viruses, but to get ahead of them.”
Final Thoughts: A World Without the Flu Shot?
While it will take time to confirm these findings in humans, the promise is clear: a universal, long-lasting influenza vaccine is within reach. In the hands of scientists like Eric Weaver and his team, the flu’s reign as a yearly menace may finally come to an end.
If the Epigraph strategy delivers on its early potential, your annual flu shot could become a relic of medical history—replaced by a powerful, enduring shield against one of humanity’s most persistent viral foes.
Reference: Erika M. Petro-Turnquist et al, Epitope-optimized vaccine elicits enduring immunity against swine influenza A virus, Nature Communications (2025). DOI: 10.1038/s41467-025-59182-7