In the complex and often disheartening world of cancer treatment, a groundbreaking development has emerged that offers a ray of hope to patients facing the bleak reality of advanced, treatment-resistant cancers. A collaborative effort between Singapore’s Agency for Science, Technology and Research’s Institute of Molecular and Cell Biology (A*STAR IMCB) and the biotechnology firm Intra-ImmuSG has led to a major milestone: the successful Phase II trial of a novel cancer immunotherapy, PRL3-zumab.
Detailed in a recent publication in Cell Reports Medicine, the therapy has shown unprecedented promise in slowing disease progression in patients with advanced solid tumors that have failed to respond to existing treatment options. This innovation not only represents a triumph in biomedical research but also ushers in a potentially paradigm-shifting approach to cancer immunotherapy.
The Science Behind PRL3-zumab
PRL3-zumab stands out in the immunotherapy landscape for its novel mechanism of action. Unlike conventional antibodies that bind to proteins on the surface of cancer cells, PRL3-zumab targets an elusive intracellular protein called PRL3 (Phosphatase of Regenerating Liver 3). This protein is overexpressed in nearly 80% of solid tumors—ranging from colorectal to gastric and ovarian cancers—yet it is virtually undetectable in healthy adult tissues.
Traditionally, antibodies have been ineffective against intracellular targets due to the impermeability of cell membranes. PRL3-zumab, however, leverages a clever biological loophole: while PRL3 typically resides inside cancer cells, it transiently appears on the cell membrane during certain stress or turnover states. PRL3-zumab seizes this fleeting window to latch onto the protein and mark the cell for destruction by the body’s immune system.
The antibody’s binding triggers two powerful immune responses: antibody-dependent cellular cytotoxicity (ADCC), where immune cells are recruited to kill the marked cancer cell, and antibody-dependent cellular phagocytosis (ADCP), where immune cells engulf and digest the target. In essence, PRL3-zumab turns stealthy, metastatic cancer cells into visible beacons for immune eradication.
Phase II Results: A Clinical Turning Point
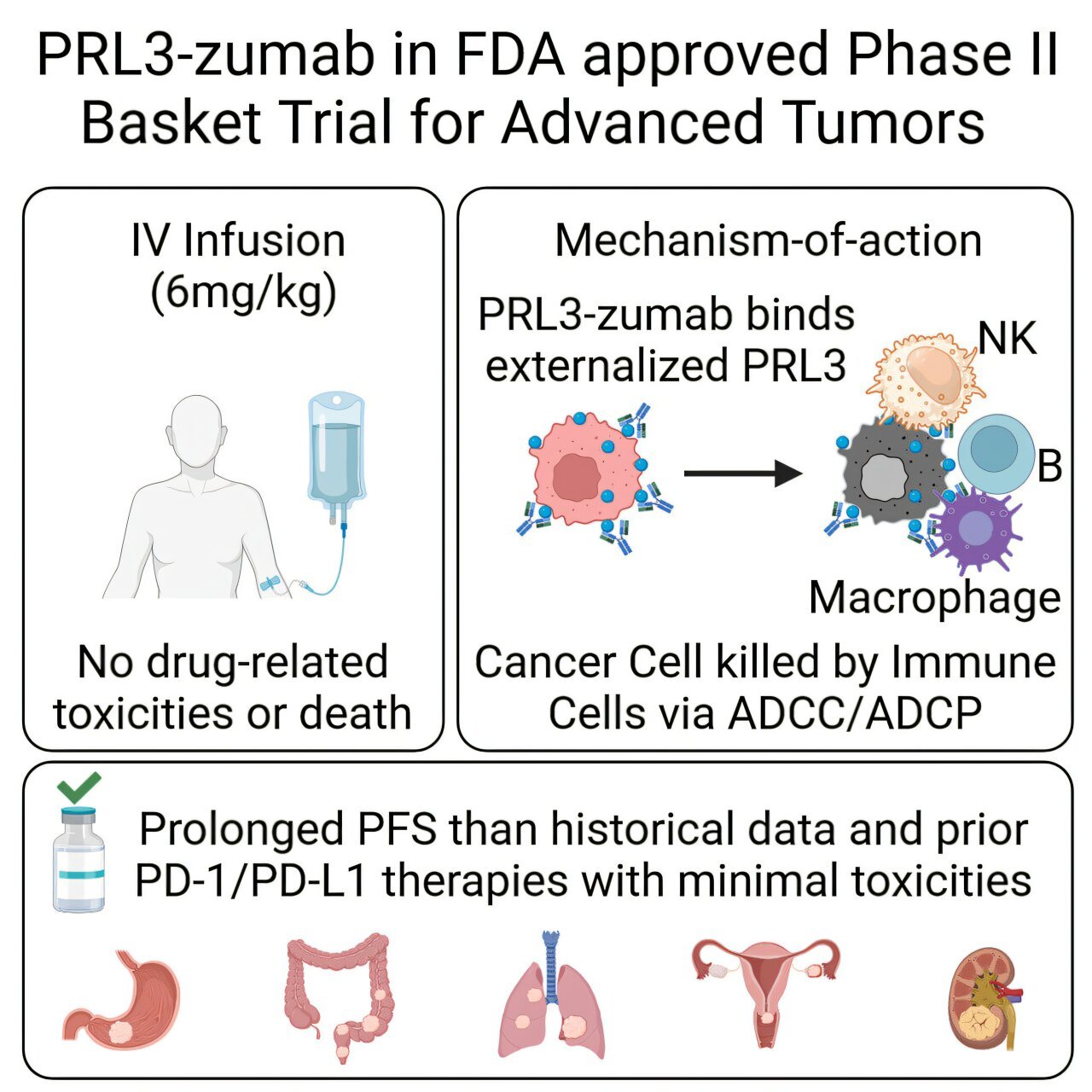
The recently completed U.S.-based Phase II trial enrolled 51 patients suffering from a variety of advanced, solid tumors—including gastric, colorectal, ovarian, and pancreatic cancers—that had not responded to conventional therapies, including checkpoint inhibitors and chemotherapies. Most of these patients had exhausted all approved lines of treatment.
Using a sophisticated analytical framework known as the Single Evaluable Patient Single Cohort (SEPSC) design, researchers compared each participant’s progression-free survival (PFS) on PRL3-zumab to their previous treatment history. The results were striking: one patient with Stage IV gastric cancer achieved disease stabilization lasting more than 13 months. This is a significant clinical improvement compared to typical post-standard therapies, where progression often resumes within just two months.
Even more compelling is the therapy’s safety profile. No serious adverse reactions or drug-related toxicities were reported, which is especially remarkable in a population with severely compromised health due to late-stage disease and prior aggressive treatments. This makes PRL3-zumab not just effective but also tolerable—a critical factor in enhancing quality of life for end-stage cancer patients.
Beyond Borders: Global Trials and Expanding Promise
While the U.S. trial primarily measured disease stabilization, companion trials in Malaysia and China have shown additional encouraging results, including measurable tumor shrinkage in a subset of patients. These findings underscore PRL3-zumab’s global potential and the therapy’s capability to offer meaningful clinical benefit across different populations and cancer subtypes.
Data from these international trials are currently under in-depth analysis, with full publications anticipated in the coming year. If early signals hold, PRL3-zumab could become the first in a new generation of antibody therapies targeting intracellular proteins—a realm long deemed “undruggable” by immunotherapy standards.
From Discovery to Breakthrough: The PRL3-zumab Origin Story
The journey of PRL3-zumab from concept to clinical reality is as compelling as its science. The story began over two decades ago with Professor Qi Zeng, a senior principal investigator at A*STAR IMCB, who first identified the PRL3 protein in 1998. At the time, PRL3’s role in cancer was largely unknown. Through years of rigorous research, Professor Zeng and her team demonstrated that PRL3 plays a key role in metastasis and chemoresistance—traits that make cancer deadly.
Recognizing the therapeutic potential of targeting PRL3, Professor Zeng co-founded Intra-ImmuSG, an A*STAR spin-off biotechnology company, to develop an antibody therapy based on her lab’s foundational discoveries. With support from public research funding and the translation ecosystem of Singapore’s biomedical landscape, PRL3-zumab advanced from the laboratory bench to first-in-human trials.
This path reflects a rare but powerful bench-to-bedside success story, a term often used in biomedical circles to describe the seamless transition of a discovery from the research lab into clinical application. It exemplifies the vision of translational medicine, where high-impact scientific discoveries directly translate into real-world therapies.
The Immunotherapy Frontier: Cracking the Code of Intracellular Targets
Immunotherapies such as checkpoint inhibitors have revolutionized cancer treatment by unleashing the immune system to attack tumors. However, these treatments only benefit a fraction of patients, largely those whose cancers exhibit certain immune-friendly profiles. For others—especially those with “cold” tumors that evade immune detection—new strategies are urgently needed.
That’s where PRL3-zumab steps in. Its ability to flag intracellular proteins offers a way to render invisible cancer cells visible to immune defenses. This innovation could pave the way for a new class of immunotherapies targeting previously inaccessible proteins inside cells. PRL3 is the first, but it may not be the last. Researchers are already exploring whether the transient membrane exposure trick used by PRL3 could be exploited for other cancer-driving proteins.
This opens a broader horizon in drug development: the expansion of antibody therapies into targets that, until now, were confined to the realm of small molecules or deemed entirely untreatable. PRL3-zumab is therefore more than a therapeutic—it’s a proof of concept for a revolutionary approach.
Real Impact for Real People
Perhaps the most moving aspect of the PRL3-zumab story is the profound impact on patient lives. The 13-month stabilization seen in a gastric cancer patient might seem like a statistic in a journal, but in the real world, it represents birthdays celebrated, time spent with family, and a chance at life after all other hope has faded.
Such stories are what drive researchers like Professor Zeng. In her words, “PRL3-zumab represents a true bench-to-bedside success story. This research product has already benefited many late-stage cancer patients and offers new hope to those with rare, aggressive cancers, helping to extend both survival and quality of life in patients who had run out of options.”
For oncologists, this represents a critical new tool in the arsenal against aggressive cancers. For biotech innovators, it’s validation that challenging conventional dogmas can lead to breakthrough innovations. And for patients and their families, it is hope—a word that often carries more weight than any scientific term.
What Lies Ahead: Regulatory and Commercial Pathways
Following the success of the Phase II trial, the logical next step will be larger, randomized Phase III trials to confirm PRL3-zumab’s efficacy across broader patient populations. If these trials replicate the earlier findings, regulatory approval could follow in the U.S., Asia, and Europe.
In parallel, Intra-ImmuSG is expected to explore commercial partnerships for manufacturing and global distribution. The company’s ambition, supported by A*STAR’s infrastructure, is to ensure that PRL3-zumab becomes accessible to patients worldwide, especially those in low- and middle-income countries where advanced cancer treatment options are limited.
Given the therapy’s safety profile and unique mechanism, there is also interest in investigating PRL3-zumab in earlier-stage cancers or in combination with other immunotherapies. Researchers believe that pairing PRL3-zumab with checkpoint inhibitors or chemotherapy might amplify its effectiveness by further sensitizing tumors to immune attack.
Conclusion: A New Era in Cancer Treatment
The development of PRL3-zumab is not just a scientific breakthrough—it’s a turning point in the evolution of immunotherapy. By proving that intracellular proteins can be targeted with antibodies, this research opens entirely new frontiers in cancer treatment. It stands as a powerful example of what sustained scientific vision, smart translational strategies, and collaborative innovation can achieve.
As the medical community eagerly awaits further trial results, one thing is certain: PRL3-zumab has not only extended lives—it has expanded the boundaries of what science believed was possible. In a landscape where so many cancer treatments eventually lead to resistance, PRL3-zumab offers something rarely seen—real, tangible, hope.
And in the fight against cancer, few things are more valuable than that.
Reference: David J. Park et al, The PRL3-zumab paradigm: A multicenter, single-dose-level phase 2 basket clinical trial design of an unconventional cancer immunotherapy, Cell Reports Medicine (2025). DOI: 10.1016/j.xcrm.2025.102120