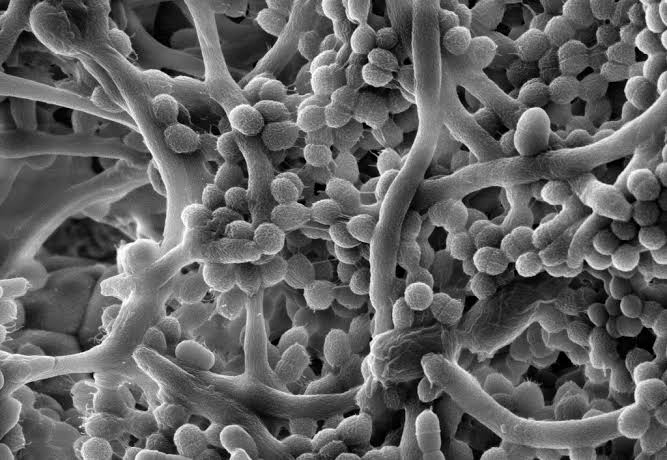
Beneath the surface of nearly every environment—be it a river rock, a hospital catheter, a kitchen sink, or a human tooth—there exists a thriving, cooperative society invisible to the naked eye. It is not built by humans, nor by insects, nor even by any multicellular organism. It is the city of the biofilm, a teeming, microscopic metropolis crafted by bacteria and other microorganisms working together for mutual survival.
To the average person, bacteria may seem like solitary invaders: lone pathogens slipping into the body to wreak havoc. But in truth, bacteria are inherently social creatures. They communicate, they organize, they defend, and—most astonishingly—they build. Biofilms are their crowning achievements, robust communities in which bacteria embed themselves in a self-produced matrix of sticky polymers. Within this gooey fortress, they gain strength in numbers, resist environmental threats, and adapt to challenges that would swiftly kill isolated cells.
Biofilms are not a fringe curiosity. They are the dominant form of bacterial life on Earth. It’s estimated that more than 80% of microbial activity occurs within biofilms, not in the free-floating or “planktonic” state often depicted in textbooks. They colonize medical devices, industrial pipelines, soil particles, and the lining of our digestive tracts. In many ways, the story of life on Earth is the story of life in a biofilm.
Understanding these microbial collectives is not just an academic pursuit—it is essential to medicine, industry, agriculture, and environmental science. Biofilms shape our health, our ecosystems, and even the evolution of life itself. To understand how bacteria survive, we must understand how they stick together.
The First Contact: How Biofilms Begin
It starts with a touch—a single bacterium landing on a surface. That surface might be a tooth, a river stone, or a surgical implant. If the conditions are right—nutrients are present, the temperature is suitable, and the surface is not too slippery—the bacterium may decide to stay.
But “decision” is the wrong word. There’s no conscious thought here. What happens instead is a cascade of molecular events. Surface receptors in the bacterium detect signals that say, “This spot looks good.” Genes activate. Flagella slow or stop. Pili—tiny hair-like appendages—reach out like grappling hooks. The bacterium sticks.
This adhesion is the first critical step in biofilm formation. It’s tentative at first, reversible. The bacterium might change its mind, detach, and float away. But if conditions remain favorable, it lays down a sticky secretion made of polysaccharides, proteins, and DNA—a slime that cements its presence. This slime is the beginning of the extracellular polymeric substance (EPS), the matrix that defines a biofilm.
More bacteria arrive. They stick, they secrete, they divide. What began as a single pioneer cell becomes a thriving colony. Communication begins. Genetic programs switch from solitary life to communal behavior. The surface is no longer just a passive object. It is now part of a living, dynamic system.
The Power of Community: Why Bacteria Cooperate
It may seem counterintuitive that such simple organisms—lacking brains, eyes, or any form of higher awareness—would act in coordinated ways. But cooperation in bacteria is not rare; it is the rule. Within a biofilm, bacteria engage in a stunning array of social behaviors.
They share nutrients. They secrete public goods—enzymes that break down complex molecules, which all members of the colony can use. They build protective barriers together. They even help each other escape threats. This cooperation is driven not by altruism but by evolution. A bacterium in a biofilm has a better chance of surviving than one drifting alone.
Communication is central to this cooperation. Bacteria use chemical signals to talk to each other, a process known as quorum sensing. As the population grows, so does the concentration of these signals. Once a threshold is reached, quorum sensing triggers group-wide changes in behavior. Biofilm formation is one of the most dramatic results. It’s as if the bacteria are taking a vote: “Do we stay here and build?” If enough of them say yes, construction begins.
But cooperation in biofilms goes deeper. There is even a kind of division of labor. Some cells focus on reproduction, others on secreting matrix, still others on producing toxins to ward off invaders. This functional differentiation resembles the organization of multicellular organisms. It blurs the line between individual and community, raising profound questions about what it means to be alive.
Architecture of Survival: The Structure of a Biofilm
To the eye of a microbiologist peering through a microscope, a mature biofilm is a marvel of organization. It’s not a uniform blob, but a landscape of towers, channels, and microcolonies. Nutrients and oxygen don’t simply seep in—they flow through carefully formed corridors like blood through veins.
This structure is not random. It emerges from the interactions between cells, the EPS matrix, and the physical environment. The matrix itself is a miracle of microbial engineering. It traps water, binds to surfaces, and resists mechanical forces. It can absorb antibiotics and neutralize immune attacks. Within this gooey world, bacteria are shielded from danger.
Importantly, the structure allows for gradients—of oxygen, pH, nutrients, and waste. Different parts of the biofilm can support different kinds of metabolism. Some areas are aerobic, others anaerobic. This heterogeneity allows bacteria to specialize, diversify, and adapt.
Even death is part of the plan. Cells in the interior may die, releasing DNA and nutrients into the matrix. This sacrificial act strengthens the biofilm as a whole. Dead cells form a kind of microbial mortar, cementing the living bricks together.
Biofilms and the Human Body: Friends and Foes
Our bodies are covered in biofilms. From our mouths to our intestines to our skin, these microbial communities are essential to health. Dental plaque is a classic example—though it becomes dangerous when left unchecked. In the gut, biofilms help digest food, train the immune system, and block pathogens. On the skin, they form a protective barrier against invaders.
But biofilms can also be deadly. Infections involving biofilms are notoriously difficult to treat. On catheters, implants, and ventilators, they cause persistent infections that resist antibiotics and immune responses. In diseases like cystic fibrosis, biofilms form in the lungs, shielding bacteria from treatment. Chronic wounds, such as diabetic ulcers, often harbor biofilms that prevent healing.
The resilience of biofilms comes down to several factors. First, the EPS matrix acts as a physical shield. Second, the interior of a biofilm is often in a slow-growing or dormant state—making antibiotics less effective, since many drugs target actively dividing cells. Third, some cells within the biofilm become “persisters”—cells that tolerate high doses of antibiotics and can reignite infection later.
Understanding and disrupting these survival strategies is a major focus of medical research. But there’s a delicate balance. Not all biofilms are bad. Many are essential to our health. The challenge is distinguishing the helpful from the harmful, and learning how to control rather than destroy.
A War of Molecules: Antibiotic Resistance and Biofilms
Antibiotic resistance is one of the greatest threats to global health, and biofilms are central to the problem. Within a biofilm, bacteria are up to 1,000 times more resistant to antibiotics than their free-floating counterparts. This resistance is not just due to the matrix or dormancy; it’s also genetic.
Biofilms are hotbeds of horizontal gene transfer. When bacteria live in close quarters, they swap genetic material with ease—plasmids, transposons, resistance genes. The matrix traps DNA fragments, allowing even unrelated species to acquire new traits. In effect, biofilms are evolutionary accelerators.
This rapid exchange fuels the rise of superbugs—bacteria resistant to multiple antibiotics. In hospitals, where surfaces and equipment become coated with biofilms, the risk is acute. Bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, and Klebsiella pneumoniae form stubborn biofilms that resist treatment and can be fatal.
The traditional approach of “kill them all with antibiotics” is increasingly failing. Instead, researchers are exploring new strategies: disrupting quorum sensing, degrading the matrix, targeting persister cells, or even harnessing bacteriophages—viruses that infect bacteria. Some scientists are looking at ancient remedies like honey and plant extracts, which have surprising anti-biofilm properties.
The war against biofilm-based infections is not one we can win by brute force. It requires precision, creativity, and a deep understanding of microbial behavior.
Biofilms Beyond Medicine: Earth’s Hidden Engineers
While much attention is focused on biofilms in health and disease, their influence stretches far beyond the clinic. In the natural world, biofilms are architects of ecosystems.
In streams and oceans, biofilms form the foundation of microbial mats that support entire food webs. They play a critical role in nutrient cycling—breaking down organic matter, fixing nitrogen, and oxidizing metals. In soils, they stabilize particles, retain moisture, and support plant roots.
One of the oldest known forms of life on Earth is the stromatolite—a fossilized biofilm that dates back over 3.5 billion years. These layered structures, created by cyanobacteria, were among the first organisms to produce oxygen through photosynthesis. They helped transform Earth’s atmosphere and pave the way for complex life.
Even in extreme environments—boiling springs, acidic caves, radioactive waste—biofilms thrive. They colonize spacecraft, survive on Antarctic rocks, and may even exist beneath the surface of Mars. Their resilience makes them both a challenge and an inspiration for astrobiology.
In industry, biofilms can be both ally and enemy. In wastewater treatment, they are essential for breaking down pollutants. In food processing, they pose a risk of contamination. In oil pipelines, they cause corrosion. Understanding their behavior helps engineers manage risk and optimize performance.
The Evolutionary Edge: Why Biofilms Matter to Life Itself
Biofilms are not a recent invention. They are likely the oldest and most successful form of life on Earth. Before plants, animals, or even single-celled eukaryotes appeared, bacteria were building biofilms. This ancient lifestyle may have been the key to survival in a hostile early Earth.
The evolution of multicellularity—a defining moment in life’s history—may have its roots in biofilm behavior. When bacteria cooperate, differentiate, and communicate, they begin to resemble simple multicellular organisms. Some species, like Myxococcus xanthus, form fruiting bodies and exhibit coordinated movement. Others undergo programmed cell death to benefit the colony.
These behaviors hint at how complex life might have evolved—not through sudden leaps, but through gradual increases in cooperation and specialization. Biofilms are nature’s laboratory for innovation. They allow bacteria to experiment with social strategies, share genes, and adapt to change.
In this light, biofilms are not just microbial conveniences. They are evolutionary engines—incubators of resilience, complexity, and novelty.
Future Frontiers: Controlling and Harnessing Biofilms
As science deepens its understanding of biofilms, new possibilities emerge. Can we design surfaces that resist unwanted biofilms but promote beneficial ones? Can we create synthetic biofilms that clean pollution, capture carbon, or produce valuable chemicals? Can we guide biofilm formation in the gut to treat disease?
Some researchers are exploring the use of biofilm-forming probiotics to displace harmful bacteria in the microbiome. Others are engineering biofilms with custom properties using synthetic biology. The goal is not just to fight biofilms but to partner with them.
There are challenges ahead. Biofilms are incredibly diverse and context-dependent. What works in one setting may fail in another. But the promise is enormous. In a world facing antibiotic resistance, environmental degradation, and chronic disease, biofilms may hold the key to smarter, more sustainable solutions.
The Secret Lives of Microbes
In the end, biofilms reveal a hidden world beneath our feet, on our skin, and within our bodies. It is a world of cooperation and conflict, survival and sacrifice, architecture and adaptation. It challenges our assumptions about what bacteria are and what life can be.
Far from being mere germs, bacteria in biofilms are builders, strategists, and survivors. They form living cities that rival our own in complexity and resilience. To understand them is to gain insight not only into microbes but into life itself.
The next time you brush your teeth, walk by a stream, or use a medical device, consider the biofilms all around you. They are not just clumps of goo. They are ancient, intelligent collectives. And they are not going anywhere.