Many vaccines work by introducing a part of a virus or its protein to the body, allowing the immune system to recognize it as foreign and produce long-lasting antibodies. These antibodies are tailored to recognize the specific virus, ensuring that when the immune system encounters the virus in the future, it can mount a faster and more effective defense.
However, groundbreaking new research from scientists at Scripps Research has uncovered an unexpected twist in the response triggered by certain HIV vaccines. After a few doses, the immune system does something unusual: instead of simply recognizing the viral protein, it begins to produce antibodies that target complexes already bound to the viral protein, in effect targeting immune molecules present on the virus’s surface.
This discovery, detailed in a January 17, 2025 paper published in Science Immunology, could hold key insights for refining and improving HIV vaccine strategies, though its full implications are still not entirely clear. For now, researchers are unsure whether this response helps or hinders the immune system’s ability to fight HIV. Nevertheless, understanding this immune behavior could be crucial for the next generation of vaccines, not just for HIV, but potentially for other infectious diseases as well.
Understanding the New Antibody Response
Most vaccines are designed to introduce a viral protein that resembles the virus in question. Once introduced into the body, the immune system recognizes this foreign protein and begins creating specific antibodies that lock onto it. These antibodies often provide protection by neutralizing the virus, rendering it incapable of infecting healthy cells in the body.
However, in the case of certain HIV vaccines, the researchers from Scripps Research found that something else occurs: after a few rounds of vaccination, some of the antibodies produced in response to the viral protein are actually directed at “immune complexes” rather than directly binding to the virus itself. These complexes are formed when the immune system’s antibodies already attached to the viral protein stimulate further immune responses, creating new antibodies that attach not to the viral protein but to the antibodies that have already bound it. This is a highly unexpected immune behavior, and scientists had not deeply studied its role in the context of HIV vaccination before this breakthrough.
Dr. Andrew Ward, the lead researcher and professor of Integrative Structural and Computational Biology at Scripps Research, explained, “These anti-immune complex antibodies have not been studied in very much depth, especially in the context of HIV vaccination. Understanding these responses could lead to smarter vaccine designs and immunotherapeutics. It’s an exciting step forward in fine-tuning antibody and vaccine-based strategies against HIV and other diseases.”
Advanced Imaging Unlocks New Insights
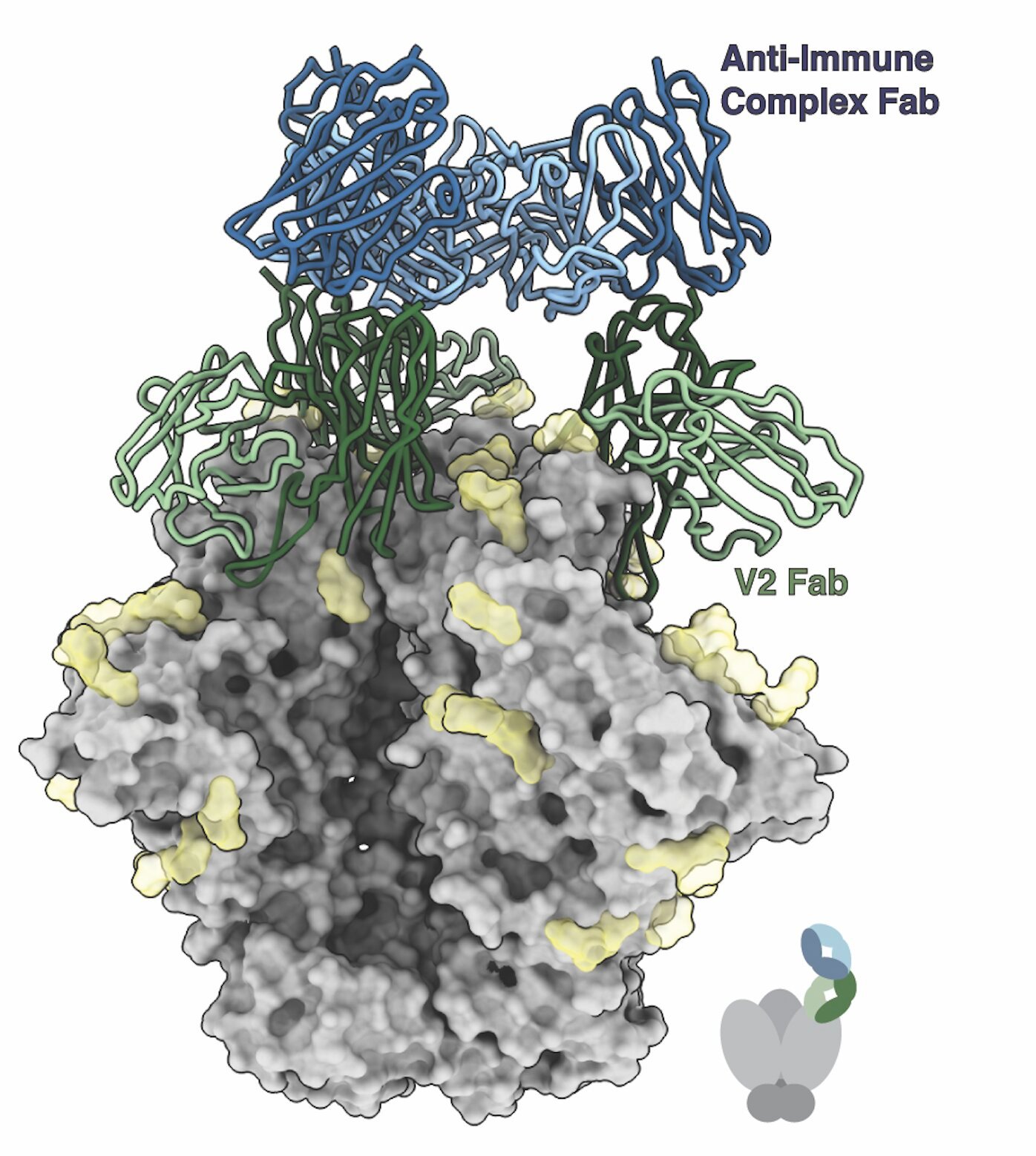
The unusual behavior of the immune system came to light when Ward’s team used advanced imaging techniques to study how antibodies evolve in response to an experimental HIV vaccine. A method developed by the lab called Electron Microscopy-Based Polyclonal Epitope Mapping (EMPEM) allowed the team to observe precisely where the antibodies bind to the virus.
What they found was surprising: many antibodies that had been produced after multiple rounds of vaccination were not directly attaching to the viral protein, as expected. Instead, these antibodies were targeting immune molecules present on the virus’s surface, but not in direct contact with the virus itself. In other words, the antibodies seemed to be targeting the immune complexes that formed when the viral protein became bound to other antibodies already in play.
Sharidan Brown, a graduate student at Scripps and the first author of the study, explained this surprising finding: “These antibodies actually make no direct contact with the viral protein. We are the first to structurally characterize this kind of antibody in the context of HIV vaccination.”
While the exact nature of these “anti-immune complex” antibodies remains unclear, their existence presents an intriguing possibility: that the immune response to the virus might evolve in a way that encourages more complex, secondary reactions after several doses of the vaccine, rather than direct neutralization of the virus.
Are These Antibodies Helpful or Harmful?
As the team continued to investigate, they discovered that these anti-immune complex antibodies often emerged after the second or third doses of the vaccine, but their impact on the immune response remains uncertain. They could potentially represent a beneficial form of immune response or they could represent an inefficient or even counterproductive reaction to the virus.
On the one hand, the immune system could be misdirected, with antibodies targeting already bound complexes rather than the virus itself. On the other hand, this immune complex formation might spur more activity against the virus by forming larger immune complexes that trigger further immune responses. As Brown explained, “We showed that these antibodies exist, but what we don’t yet know is how they shape the immune response. They could be detrimental because they are not directly neutralizing the virus, but they could lead to larger immune complexes which actually spur more activity against the viruses and infected cells in ways that we don’t fully understand.”
Understanding how these anti-immune complex antibodies might influence the body’s overall immune strategy is crucial, as it could have implications for the design of more effective HIV vaccines. If experiments show these antibodies are not helpful, there could be efforts to tweak the vaccine’s formulation to minimize or avoid them.
Implications for HIV Vaccine Development
The discovery raises key questions about the current strategies for developing vaccines for HIV, a virus that has long eluded effective vaccination despite concerted global efforts. Currently, some HIV vaccines focus on generating neutralizing antibodies that bind directly to the virus to block its ability to infect cells. If future research finds that immune complexes generated during vaccination are not contributing to the fight against the virus, scientists may explore ways to modify vaccine schedules to promote stronger direct antibody responses.
For example, one possibility is to modify the vaccine’s booster doses. By adjusting the timing, frequency, or formulation of subsequent vaccine doses, it could be possible to reduce the formation of these anti-immune complex antibodies and instead encourage more direct virus-neutralizing activity.
The research could also lead to the incorporation of diverse viral antigens in the vaccine schedule. As Brown notes, “Minor changes between each dose could create just enough diversity that you don’t produce antibodies against antibodies.” This approach may reduce the tendency to produce immune responses that target immune complexes rather than the virus itself.
Broader Implications for Vaccine Development
The implications of these findings go beyond just the pursuit of an HIV vaccine. The mechanism uncovered by Scripps Research could extend to other vaccines, especially those requiring multiple doses or exposure to viral proteins. Understanding how immune complexes evolve in response to vaccines, and whether or not they contribute to effective immunity, could ultimately lead to novel strategies for a wide array of vaccines.
The possibility of generating more targeted, efficient immune responses by tweaking vaccine schedules or formulations could be particularly important for diseases like malaria, tuberculosis, or even more elusive viral diseases like dengue and Zika. Improved vaccine strategies would not only benefit HIV research but could make a significant impact on public health worldwide by enabling better responses to a wide range of infectious diseases.
Conclusion
The discovery of anti-immune complex antibodies in HIV vaccination, as revealed by the Scripps Research team, offers promising new insights that could transform vaccine design. While the exact role of these antibodies remains uncertain, their potential to influence the immune response presents both challenges and opportunities. If further research demonstrates that these antibodies hinder direct neutralization of the virus, future HIV vaccine strategies could focus on minimizing such responses, adjusting vaccine schedules, or varying antigen formulations. This groundbreaking finding may not only improve HIV vaccines but could also extend to other infectious diseases, providing new avenues for more effective vaccines globally. By fine-tuning immune responses, these advances could significantly enhance our ability to prevent and treat a wide range of diseases, making a lasting impact on global health. Ultimately, understanding these immune reactions is a critical step toward smarter vaccine development.
Reference: Sharidan Brown et al, Anti-Immune Complex Antibodies are Elicited During Repeated Immunization with HIV Env Immunogens, Science Immunology (2025). DOI: 10.1126/sciimmunol.adp5218. www.science.org/doi/10.1126/sciimmunol.adp5218