For most of human history, the genome—the complete set of instructions that makes us who we are—was an impenetrable mystery. Hidden deep within the nucleus of every cell, DNA carried the secrets of life, disease, and evolution, locked in a language no one could decipher, let alone change. Doctors could treat symptoms. Scientists could observe mutations. But the genome itself remained untouchable—a book we could read but never edit.
Then came gene editing.
What began as a trickle of genetic discovery has turned into a tidal wave of breakthroughs. From the groundbreaking simplicity of CRISPR to the elegant precision of base and prime editing, humanity has developed the tools not only to read the language of life, but to rewrite it. We can now correct deadly mutations, engineer crops to survive climate change, design super-targeted cancer treatments, and even alter the DNA of future generations.
These advances are more than scientific achievements—they are a fundamental shift in how we understand life and disease. Gene editing is not just medicine; it is transformation at the most basic biological level. It is hope for the incurable, tools for the impossible, and answers to questions we’ve been asking since the dawn of biology.
But with this unprecedented power comes profound responsibility. What does it mean to edit human embryos? Who decides what is “normal” or “desirable”? How do we balance innovation with ethics, progress with precaution?
This article explores ten of the most revolutionary advances in gene editing—each a milestone in our journey from decoding life to designing it. These are not distant dreams; they are happening now, in labs, in clinics, and in living cells. Together, they mark the dawn of a new era in science—a time when the limits of biology are being rewritten, one letter at a time.
1. CRISPR-Cas9: The Molecular Scissors That Changed Everything
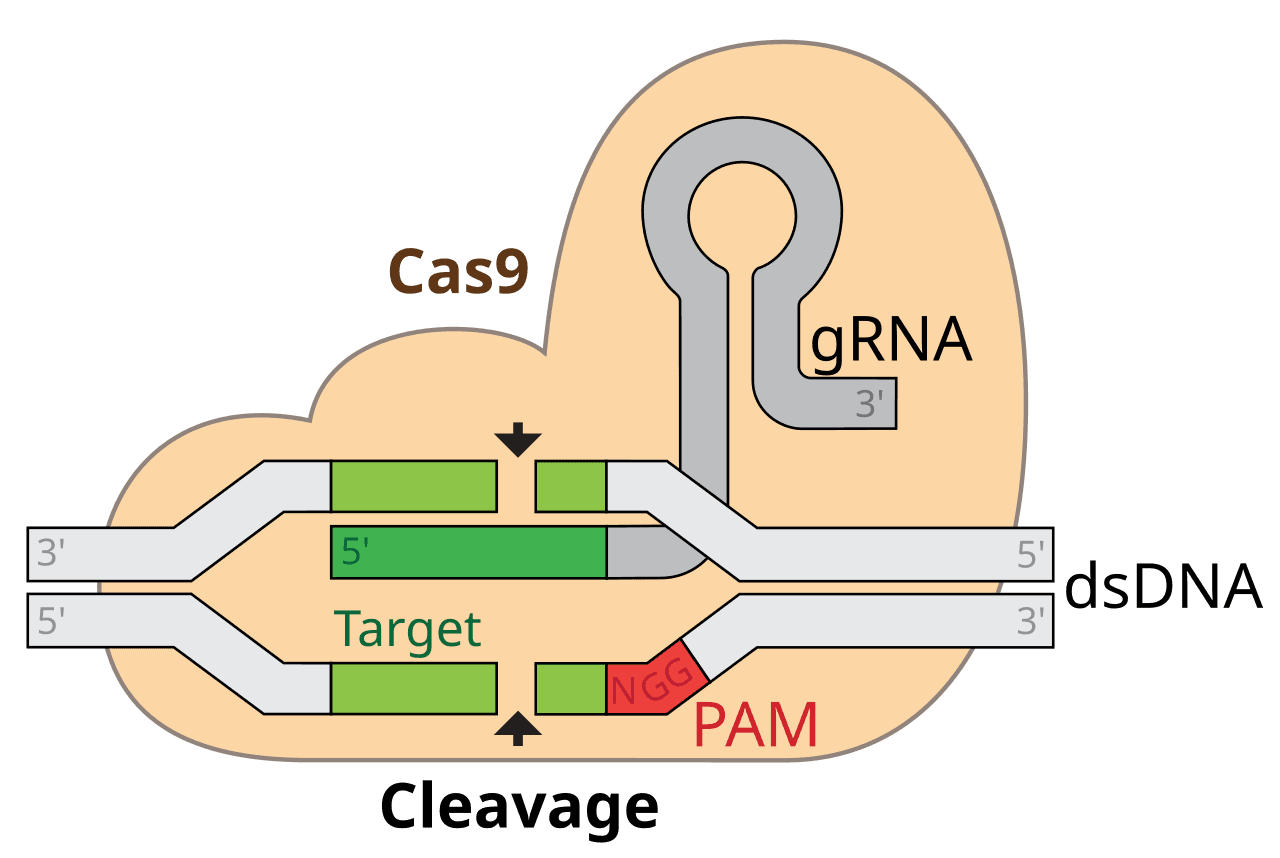
When Jennifer Doudna and Emmanuelle Charpentier unveiled CRISPR-Cas9 in 2012, they didn’t just introduce a new tool—they sparked a genetic revolution. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) was originally part of a bacterial immune system, used by microbes to chop up invading viruses. What researchers realized was that these molecular “scissors” could be repurposed to snip DNA at almost any location.
Cas9 is the enzyme that does the cutting. Guided by a piece of RNA that matches a specific genetic sequence, it can zero in on a target and make a precise incision. Scientists can then delete, replace, or insert genes—opening the door to treating genetic diseases at their root.
What makes CRISPR-Cas9 so revolutionary is its simplicity, affordability, and efficiency. Gene editing that once cost hundreds of thousands of dollars and took months can now be performed in days for just a few hundred dollars. It democratized gene editing, letting labs around the world join the frontier.
The results have been astonishing. Labs have edited human embryos, engineered disease-resistant plants, and even attempted to bring extinct species back to life. In 2020, Doudna and Charpentier were awarded the Nobel Prize in Chemistry for their work—a recognition not just of a breakthrough, but of a tool with the potential to rewrite life.
2. Base Editing: Correcting Mutations Without Breaking DNA
While CRISPR-Cas9 is powerful, it can be a bit rough. Cutting DNA can trigger unwanted consequences—especially when the cell tries to repair the break and makes mistakes. That’s where base editing comes in: a gentler, more precise way to correct individual DNA letters.
In 2016, Harvard’s David Liu introduced the concept. Instead of cutting the DNA strand, base editors chemically convert one base to another. Think of it as spell-check for the genome. If you imagine the genome as a 3-billion-letter book, base editing can change a typo like “sickl” to “sickle” with surgical precision—without tearing the page.
The technique currently allows A•T to G•C or C•G to T•A conversions. This might seem narrow, but it covers a huge range of mutations. In fact, around 60% of known genetic disorders stem from single-point mutations—tiny errors that base editing could fix.
In 2023, researchers used base editing in a human clinical trial to treat a rare form of cholesterol disorder (familial hypercholesterolemia), offering hope for millions. This method promises not just to cure disease, but to do it with almost no collateral damage.
3. Prime Editing: The Word Processor of DNA
Where base editing is spell-check, prime editing is like a full-featured word processor for the genome. Developed in 2019 by the same David Liu who gave us base editing, prime editing is one of the most advanced gene editing systems yet.
Here’s how it works: a “prime editor” enzyme combines a modified Cas9 protein (which nicks rather than cuts the DNA) with a reverse transcriptase enzyme and a specially designed RNA template. This template includes the desired genetic change—whether it’s a single letter or a longer segment of DNA.
Unlike CRISPR-Cas9, which relies on the cell’s natural repair mechanisms, prime editing writes in the change directly. It’s more versatile, more accurate, and far less likely to cause errors.
This means prime editing could potentially correct up to 89% of known genetic mutations—including those that CRISPR and base editing struggle with. It’s still in its early stages, but in lab tests, prime editing has already been used to correct mutations responsible for Tay-Sachs disease, sickle cell anemia, and more.
The vision? A future where every inherited disease could be rewritten, base by base, into health.
4. In Vivo Gene Editing: Editing DNA Inside the Body
One of the holy grails of gene editing is in vivo therapy—changing genes directly inside a patient’s body. Until recently, most gene editing had to be done ex vivo: cells were taken from the body, edited in a lab, and reintroduced. That method works for certain diseases but not for conditions affecting tissues like the brain, liver, or muscles.
The breakthrough came in 2021, when Intellia Therapeutics and Regeneron announced the first successful in vivo CRISPR treatment in humans. They treated a rare liver disorder called transthyretin amyloidosis (ATTR) by delivering CRISPR-Cas9 via lipid nanoparticles directly into the bloodstream. The gene editing happened inside the liver cells—and it worked. Levels of the harmful protein dropped by over 90%.
This was a landmark moment: gene editing as a one-time injection.
It’s just the beginning. In vivo editing could target countless conditions—cystic fibrosis in the lungs, muscular dystrophy in the muscles, or even neurodegenerative diseases like Huntington’s and Alzheimer’s in the brain. Delivery remains a major challenge, but nanotechnology and viral vectors are advancing quickly.
The idea that we can now “patch” our genetic code while we sleep? It sounds like science fiction—but it’s becoming science fact.
5. Epigenome Editing: Rewriting the Software of the Cell
The genome is the hardware. The epigenome is the software that tells the genome what to do. Epigenetic marks—like methyl groups or acetyl tags—don’t change the DNA itself, but they control which genes are turned on or off.
In recent years, scientists have learned to edit this software with extraordinary precision. Epigenome editing uses modified versions of CRISPR proteins (without the DNA-cutting ability) fused to enzymes that add or remove epigenetic marks. These molecular tools can dial gene activity up or down—without altering the sequence.
Why is this powerful? Because many diseases aren’t caused by mutations, but by misregulation. Cancer, depression, metabolic disorders—all can be triggered by genes being expressed at the wrong time or in the wrong place. Epigenome editing lets us reset those patterns.
In 2022, researchers used CRISPR-based epigenetic tools to silence a gene in the brains of mice that’s involved in anxiety. The treatment had long-lasting behavioral effects—without changing a single DNA letter. It’s a glimpse of a future where we can adjust gene expression with the same flexibility as tuning a radio.
6. Multiplex Gene Editing: Engineering Entire Genetic Networks
Gene editing started with changing one gene at a time. But biology is not so simple. Most diseases—especially cancers, autoimmune conditions, and developmental disorders—result from interactions between multiple genes.
That’s where multiplex editing comes in. With CRISPR’s versatility, scientists can now target several genes simultaneously. They can delete one, enhance another, and insert a third—all in one go.
This technique is already revolutionizing synthetic biology. Researchers have used multiplex editing to create yeast that produce rare opioids, rice that fixes its own nitrogen, and pigs with organs suitable for human transplants.
In 2023, scientists used multiplex CRISPR to engineer immune cells (CAR-T cells) with up to 20 different edits—making them more powerful cancer killers, more stealthy to the immune system, and more persistent in the body.
The ability to rewrite genetic networks—rather than just single switches—could enable cures for polygenic diseases, optimize crops for climate resilience, and even engineer microbial ecosystems to clean up the environment.
7. Gene Drives: Reprogramming Evolution
Imagine if a genetic trait could be passed to nearly all of an organism’s offspring, not just half. That’s the concept behind gene drives—genetic elements that copy themselves into both chromosomes of an organism, ensuring near-universal inheritance.
Using CRISPR, scientists can build gene drives that spread specific traits rapidly through populations. For example, they’ve created mosquitoes that pass on genes rendering them infertile or resistant to malaria. Releasing just a few edited individuals could, in theory, crash entire disease-carrying populations.
The potential is huge—but so are the risks. Gene drives could transform ecosystems, with unpredictable consequences. There are deep ethical questions: Who decides which species to edit? What if a gene drive jumps to another population?
As a result, gene drive experiments are tightly regulated and mostly confined to labs. Still, they represent one of the boldest applications of gene editing—a tool not just for treating disease, but for reshaping the biosphere.
8. RNA Editing: The Fast Lane to Genetic Therapy
DNA is the library. RNA is the working copy. What if we could edit RNA instead of DNA?
RNA editing is emerging as a faster, reversible, and potentially safer way to manipulate genes. One powerful tool is ADAR (adenosine deaminase acting on RNA)—an enzyme that can change individual RNA letters, effectively fixing mutations at the RNA level.
In 2023, researchers used RNA editing in primates to temporarily treat Rett syndrome, a devastating neurological disorder. Because RNA naturally degrades over time, the edits faded—making the technique ideal for short-term therapies, like treating infections or regulating immune responses.
RNA editing avoids the risks of permanent DNA changes. It’s also fast: new treatments can be designed and deployed in weeks, not years. Companies like Shape Therapeutics and ProQR are already developing RNA editors for liver disease, cystic fibrosis, and genetic blindness.
While DNA editing rewrites the book, RNA editing just marks up the copy—and sometimes, that’s all you need.
9. Organoid and Embryo Editing: Rewriting Life’s Blueprint
In the lab, scientists can now grow miniature versions of organs—organoids—from stem cells. These 3D tissue cultures mimic the architecture and function of lungs, brains, livers, and more. Gene editing within organoids is opening a window into human development like never before.
Researchers can model diseases, test drugs, and experiment with genetic corrections—all without touching actual patients. In 2024, scientists used CRISPR to correct a mutation in kidney organoids derived from a patient with polycystic kidney disease. The gene-edited organoids functioned normally—proving that personalized, lab-grown therapies could soon be possible.
Then there’s embryo editing—perhaps the most controversial frontier. In 2018, Chinese scientist He Jiankui stunned the world by announcing he had edited the genomes of twin girls using CRISPR. He claimed the edits would make them resistant to HIV.
The scientific community reacted with shock and outrage. The ethics were murky, the risks unclear, and the consent—given on behalf of unborn children—was deeply problematic.
Still, the science marches on. Researchers continue to explore embryo editing under tight regulation. The goal is not designer babies, but prevention of devastating inherited disorders. The power to intervene in the earliest moments of life forces us to confront profound questions: What should we edit? And who gets to decide?
10. Personalized Gene Editing Therapies: The Birth of Genetic Medicine
In 2024, a 13-year-old girl with sickle cell anemia received a one-time CRISPR treatment customized to her genetic mutation. A year later, she was symptom-free. This wasn’t a clinical trial—it was medicine.
The age of personalized gene editing is here.
Advances in sequencing, AI, and bioinformatics are enabling custom therapies built for individual patients. No more one-size-fits-all treatments. Each genetic profile, each mutation, each cell type can be addressed precisely.
Startups like Verve Therapeutics, Beam Therapeutics, and Editas Medicine are developing bespoke gene editing drugs for conditions ranging from heart disease to rare metabolic disorders. Therapies are moving out of labs and into clinics—transforming how we think about disease.
Someday soon, you might walk into a hospital, get your DNA sequenced, and receive a personalized gene-editing treatment before you leave. The idea of “inherited” disease may soon become obsolete.
Conclusion: Editing the Story of Life
In just over a decade, gene editing has evolved from a lab curiosity to a tool that touches almost every aspect of biology and medicine. We now have the power to correct mutations, rewrite entire networks of genes, and even alter species. We are editing not just our future, but the blueprint of life itself.
With that power comes responsibility. The ethical, ecological, and societal implications are vast. What diseases should we cure? What traits should we enhance? What risks are we willing to accept?
Gene editing holds unimaginable promise. But it also challenges us to be wise, compassionate, and cautious. As we step into a future where life is no longer just inherited, but engineered, the question is no longer what we can do—but what we should do.
One thing is certain: the revolution has already begun.