They are not alive in the traditional sense. They cannot move, eat, or breathe. They have no metabolism and no will of their own. Yet viruses, those microscopic particles on the edge of life, are among the most powerful biological forces on Earth. They shape ecosystems, drive evolution, and, as we’ve seen in recent years, have the power to halt societies in their tracks. But how do these invisible entities multiply? How does a tiny strand of genetic material inside a protein shell hijack the molecular machinery of a living cell and turn it into a viral factory?
The answer lies in two distinct yet interwoven strategies: the lytic cycle and the lysogenic cycle. These replication paths are not merely mechanisms of reproduction—they are intricate biological dramas that reveal the essence of viral existence. Understanding them is not only key to grasping the nature of viruses but also illuminates the fine line between life and death at the microscopic level.
What Exactly Is a Virus?
Before diving into the mechanics of viral replication, it’s essential to understand what a virus truly is. At its core, a virus is a genetic parasite—a tiny package of nucleic acids (DNA or RNA) encased in a protein coat called a capsid. Some viruses have an outer lipid envelope, derived from the membranes of their host cells. But stripped to its essentials, a virus contains just enough instructions to reproduce—provided it finds a suitable host.
Unlike living cells, viruses cannot replicate independently. They have no ribosomes to build proteins, no mitochondria to generate energy, and no cellular machinery to transcribe or translate their genetic code. Instead, they exist in a twilight zone: inert outside a host, terrifyingly efficient inside one. Once they infect a cell, however, they unleash a cascade of molecular manipulation that turns their host into a puppet—and sometimes into a ticking time bomb.
The Host-Cell Invasion
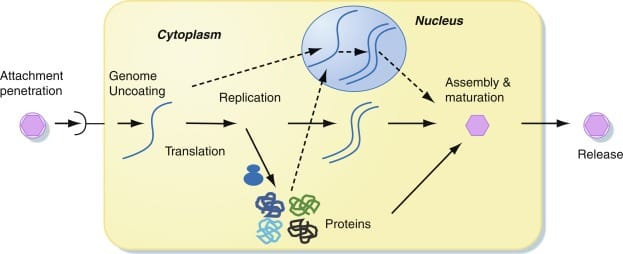
The first step in any viral replication strategy is infection. The virus must gain access to a host cell, and to do this, it must recognize and bind to specific receptors on the cell’s surface. This specificity is why most viruses only infect certain types of cells or organisms. The flu virus, for instance, targets respiratory cells in humans, while bacteriophages—the viruses that prey on bacteria—recognize unique molecules on bacterial surfaces.
Once a virus attaches to its target, it must deliver its genetic material inside. Some viruses inject their DNA or RNA directly through the cell membrane. Others are engulfed via endocytosis, slipping in like a Trojan horse. In some cases, particularly with enveloped viruses like HIV or SARS-CoV-2, the viral membrane fuses with the cell’s membrane, releasing its contents into the cytoplasm.
From here, the viral genome begins its subversive campaign—either commandeering the host’s machinery for immediate replication or biding its time, hiding within the host’s DNA. The path it takes next determines whether the cell survives, transforms, or dies.
The Lytic Cycle: Viral Blitzkrieg
The lytic cycle is the viral equivalent of shock and awe. Once a virus enters a cell and commits to the lytic pathway, it moves rapidly and ruthlessly. Its sole goal is to produce as many new viral particles as possible, as quickly as possible—even if it means destroying the host in the process.
Once inside, the virus hijacks the host’s ribosomes, enzymes, and energy to replicate its genome and synthesize viral proteins. These components then self-assemble into new virions—individual virus particles capable of infecting new cells. It’s an assembly line of microscopic proportions, churning out thousands of copies in a matter of hours.
As the virions accumulate, the host cell becomes bloated with viral progeny. Eventually, the cell can no longer contain them. In many cases, special viral proteins disrupt the host’s plasma membrane or cell wall, leading to lysis—the violent rupture of the cell. The newly minted virions spill out into the surrounding environment, ready to infect neighboring cells and begin the cycle anew.
This cycle of invasion, replication, and destruction is typical of many bacteriophages, such as T4 phage, which infects Escherichia coli. But it’s also used by some animal viruses, like adenoviruses, which cause respiratory infections. In every case, the lytic cycle is a brutal yet effective strategy—a short-term gain achieved through the host’s demise.
The Lysogenic Cycle: A Silent Infiltration
Not all viruses are so immediately destructive. Some take a more subtle, long-term approach—integrating into the host’s genome and waiting patiently for the right moment to emerge. This is the lysogenic cycle: a stealth operation in which the virus becomes part of the host cell’s DNA, lying dormant until triggered to activate.
In this cycle, once the viral genome enters the host cell, it does not immediately begin replication. Instead, it becomes incorporated into the host’s chromosome, forming what’s known as a prophage (in the case of bacteriophages) or a provirus (in animal cells). In this integrated state, the viral DNA is passively replicated alongside the host’s own genetic material every time the cell divides.
For the host, this arrangement may appear benign. The cell continues to function normally, unaware of the lurking intruder. But the viral genes may subtly influence host behavior, or even provide advantages. In some cases, the presence of viral DNA can alter the cell’s phenotype, enhance its resistance to stress, or even increase its virulence—turning an ordinary bacterium into a dangerous pathogen.
The lysogenic cycle can persist indefinitely. But it is not permanent. Under certain conditions—often triggered by stress, UV light, or chemical exposure—the dormant virus reactivates. The prophage excises itself from the host genome and enters the lytic cycle, initiating the rapid production of new virions and the eventual destruction of the host.
A Dance of Strategy: Lytic vs Lysogenic Decision-Making
For viruses that can follow either pathway, the choice between lytic and lysogenic replication is more than random chance—it’s a calculated strategy. Molecular switches within the viral genome, influenced by environmental signals, guide this decision.
In the case of the well-studied lambda phage (λ phage), which infects E. coli, two key proteins—Cro and CI—play opposing roles. CI promotes lysogeny by repressing lytic genes, while Cro does the opposite. The concentration and stability of these proteins determine the phage’s fate.
If the host cell is healthy and resources are abundant, the virus may opt for the lytic cycle, maximizing its reproductive output. If the environment is harsh or the cell is stressed, the virus may choose lysogeny, lying low until conditions improve.
This dynamic balance reflects an evolutionary wisdom: sometimes survival depends on patience. The lysogenic cycle provides a way for the virus to endure lean times, spreading quietly as its host multiplies, ready to awaken when the moment is right.
Lysogeny’s Hidden Powers: Genetic Change and Evolution
While lysogeny may seem passive compared to the lytic cycle’s aggression, it has profound consequences—both for individual cells and for evolution as a whole. Integrated viral DNA can alter the genetic landscape of its host in powerful ways.
One striking example is lysogenic conversion, in which the presence of a prophage changes the phenotype of the host bacterium. Some of the most infamous bacterial toxins—including those from Corynebacterium diphtheriae (diphtheria), Vibrio cholerae (cholera), and Clostridium botulinum (botulism)—are encoded by prophage genes. Without their viral passengers, these bacteria would be harmless.
In this way, viruses act as genetic vectors, transferring genes between organisms and introducing new traits. This phenomenon, known as horizontal gene transfer, plays a vital role in microbial evolution. Over millions of years, viruses have helped shape the genomes of all life—bacteria, animals, even humans.
In fact, the human genome contains remnants of ancient viral infections. So-called endogenous retroviruses—viral sequences that integrated into germ cells and were passed on to offspring—make up nearly 8% of our DNA. Some of these ancient genes have even been co-opted for human functions, including placental development. We are, in a very real sense, part virus.
Lytic and Lysogenic Cycles in Human Disease
Understanding the difference between lytic and lysogenic cycles is not just an academic exercise—it has real-world implications, especially in virology and medicine.
Some human viruses, like herpes simplex virus (HSV), follow a latent strategy similar to lysogeny. After an initial infection, HSV retreats into nerve cells, where it remains dormant for years. Periodically, it reactivates and causes new outbreaks. Similarly, HIV, the virus that causes AIDS, integrates into the host’s DNA as a provirus and can remain latent for years before becoming active.
This latency poses major challenges for treatment. Antiviral drugs that target actively replicating viruses are often useless against latent infections. The virus is hidden, silent, and untouchable—waiting until the immune system weakens or other triggers cause it to reactivate.
On the other hand, some viruses remain lytic throughout, like the rhinoviruses responsible for the common cold. These viruses replicate rapidly and cause acute symptoms, but are typically cleared by the immune system without establishing long-term infection.
Cancer-causing viruses, such as human papillomavirus (HPV) and Epstein-Barr virus (EBV), often use lysogenic strategies to insert their genomes into host cells. The integration of viral DNA can disrupt normal cellular controls and lead to uncontrolled growth. Understanding these mechanisms is essential for developing vaccines, antiviral drugs, and cancer therapies.
The Evolutionary Arms Race
Viruses and their hosts are locked in an eternal evolutionary arms race. Every strategy a virus employs—whether it’s lytic aggression or lysogenic stealth—is met with a countermeasure from the host. Bacteria, for instance, have developed CRISPR-Cas systems, which act as a kind of immune memory, recognizing and destroying invading viral DNA.
Viruses respond by mutating their genomes or evolving anti-CRISPR proteins to bypass these defenses. Some even incorporate pieces of host DNA into their own genomes, further blurring the line between invader and host.
This evolutionary battle is ongoing, and it shapes not only the lives of viruses and their hosts, but also the structure of ecosystems and the course of evolutionary history. From the deep sea to the human body, viruses play a central role in the web of life—silent, powerful, and often misunderstood.
Future Frontiers in Viral Replication
As our understanding of viral replication deepens, so do the possibilities for innovation. Scientists are now exploring how to harness viruses for beneficial purposes—using bacteriophages to treat antibiotic-resistant infections, designing viral vectors for gene therapy, and even programming viruses to target cancer cells selectively.
By decoding the intricacies of the lytic and lysogenic cycles, researchers can engineer safer, more efficient viruses for medicine and biotechnology. The ability to control when a virus goes lytic or remains latent could transform treatments for genetic diseases, infections, and cancer.
Moreover, emerging research into phage therapy—the use of lytic bacteriophages to combat bacterial infections—may provide a powerful alternative to traditional antibiotics, especially as antibiotic resistance continues to rise.
A Tale of Two Strategies
The lytic and lysogenic cycles are not just mechanisms of replication—they are expressions of viral intelligence. They reveal the stunning adaptability of viruses, their ability to toggle between patience and aggression, dormancy and destruction.
In the lytic cycle, we see the raw power of replication—fast, efficient, and lethal. In the lysogenic cycle, we witness subtlety and strategy—a long game of genetic infiltration and silent persistence. Together, they tell the story of viruses as master manipulators of life, capable of influencing organisms, shaping evolution, and even rewriting the rules of biology itself.
The Deepest Lesson: Life at the Edge
Studying viral replication forces us to confront profound questions. What does it mean to be alive? Can something that does not grow, eat, or breathe still possess a form of agency? Viruses straddle the boundary between living and non-living, between inert particles and dynamic forces. Their replication cycles—lytic and lysogenic—are not just scientific curiosities, but windows into the fundamental principles of life.
In every cell they infect, viruses remind us that even the smallest, simplest forms can wield enormous power. And as we learn to understand, predict, and perhaps even control their behavior, we also gain deeper insights into our own biology, our vulnerabilities, and our shared molecular ancestry.
In the end, the lytic and lysogenic cycles are not merely the lifeblood of viruses—they are the language of survival, adaptation, and the perpetual dance between creation and destruction.