In a scientific breakthrough with the potential to transform global medicine and biotechnology, a new study reveals that ultra-simple molecules—just three amino acids long—can mimic nature’s own strategy for protecting delicate proteins from environmental stress. The findings, published in Nature Materials by researchers at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC), unveil a minimalist yet powerful approach to stabilizing biomolecules like vaccines and therapeutic proteins—potentially without the need for refrigeration.
Led by Professor Rein Ulijn, founding director of the CUNY ASRC Nanoscience Initiative and distinguished professor of chemistry at Hunter College, the research explores how short peptides can create dynamic, reversible compartments that protect sensitive proteins during extreme conditions such as drying. Upon rehydration, the peptides release the proteins intact, offering a promising new alternative to conventional cold-chain storage.
Cracking Nature’s Code with Three Amino Acids
At the heart of this study lies a compelling question: can we replicate the biological resilience seen in creatures like tardigrades—microscopic organisms famous for surviving in the harshest environments on Earth?
“Tardigrades can endure near-total dehydration by forming protective molecular compartments,” Ulijn explains. “They manage to preserve their biological machinery even in deep space or boiling temperatures. Inspired by that, we asked: could we design a minimal synthetic system to do the same for sensitive proteins?”
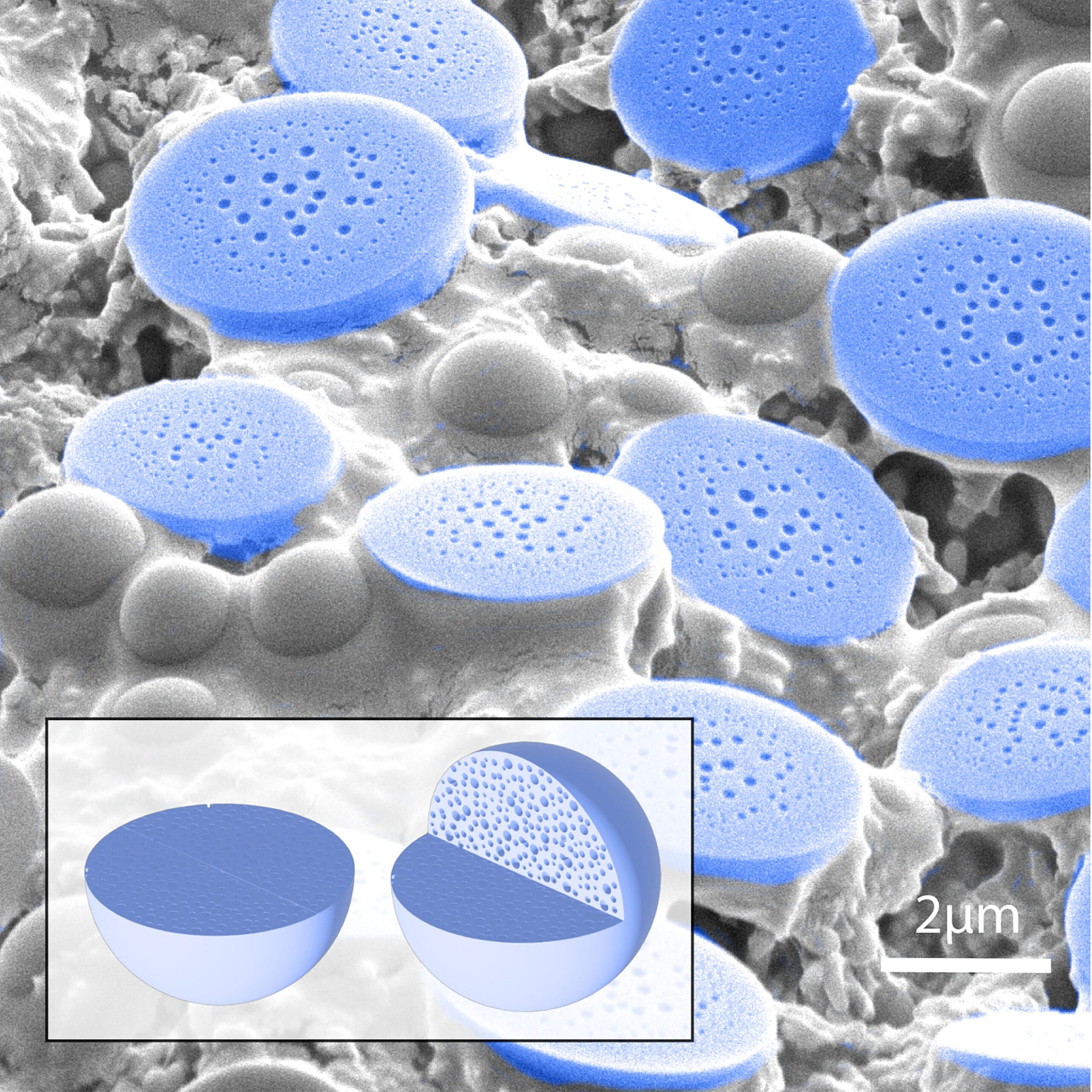
The answer, remarkably, turned out to be yes. By using tripeptides—chains of just three amino acids—the research team demonstrated that these molecules can spontaneously self-organize into disordered but functional compartments. These compartments form through a process called liquid–liquid phase separation, a mechanism commonly used by living cells to protect vulnerable components under stress.
As the peptides dry, they undergo phase separation to form porous microparticles that effectively encapsulate proteins. These tiny, sponge-like structures are strong enough to shield their cargo from environmental damage, yet flexible enough to release them safely when rehydrated.
Phase Separation: Nature’s Molecular Shelter
Phase separation is a biological process in which cells temporarily separate certain proteins and molecules into compartments without needing membranes. This allows cells to protect essential proteins from damage during stress, such as heat or dehydration, and then return to normal once conditions improve.
In the lab, Ulijn and his colleagues showed that this same process could be mimicked with a simple drying step. Tripeptides mixed with proteins in water formed reversible assemblies as the solution dried. These assemblies solidified into porous structures that trapped and protected the proteins inside.
When water was reintroduced, the peptide cages dissolved, releasing the proteins with their structure and function fully preserved. The process required no complex chemistry, no refrigeration, and no additives—just peptides, proteins, and water.
Why This Matters: A New Era for Protein Stabilization
This discovery could have profound implications for global health. One of the biggest challenges in medicine today is the need to keep vaccines, insulin, antibodies, and other therapeutic proteins cold during storage and transport. In many parts of the world—particularly in low-income or remote regions—this cold chain is expensive, unreliable, or nonexistent.
“This work opens up a completely new way to think about protein preservation,” said Ulijn. “With the right peptide materials, we might not need elaborate formulations or refrigeration anymore. That’s a game-changer.”
Vaccines and biological medicines are notoriously fragile. Exposure to heat, light, or drying can render them useless, often leading to waste or inaccessibility in regions that need them most. The idea that a synthetic material made of just three amino acids could offer reliable, low-tech protection is nothing short of revolutionary.
From the Lab to the World: Applications and Future Impact
The immediate applications of this work could be in the development of temperature-stable vaccines. Imagine a COVID-19 vaccine that could be shipped and stored at room temperature—without fear of spoilage. This could dramatically expand access to immunizations, reduce reliance on cold-chain infrastructure, and slash distribution costs.
But the potential goes far beyond vaccines. Enzymes used in diagnostics, industrial processes, and even research labs could benefit from easier storage. The approach could also lead to smart biomaterials—substances that respond to environmental cues by releasing proteins only when needed.
“In addition to the practical uses, this also offers new scientific insights,” Ulijn added. “We’re seeing a form of supramolecular assembly that’s both minimal and functional. It’s a new way for peptides to organize, and it expands our understanding of material science at the molecular level.”
Less Is More: The Beauty of Minimal Design
One of the most striking aspects of the study is its simplicity. Many biomolecular stabilization methods rely on intricate chemical formulations or bulky synthetic polymers. In contrast, the tripeptides used in this study are short, naturally occurring chains made from amino acids—the basic building blocks of life.
These minimalist materials require no special equipment to assemble. They self-organize under basic conditions, use water as their medium, and disassemble just as easily. In a world where sustainability and cost-effectiveness are becoming more important than ever, the beauty of this low-tech, high-impact solution is hard to ignore.
It’s also highly adaptable. By changing the composition of the tripeptides, scientists can fine-tune how they behave, what proteins they interact with, and how fast they release their cargo. This tunability opens doors to custom-designed preservation systems for different classes of biomolecules.
A Bridge Between Biology and Technology
What makes this study resonate on a deeper level is the way it connects two worlds: the elegant strategies of nature and the precise demands of modern science. In observing how organisms like tardigrades survive in the harshest environments, researchers have reverse-engineered a method that could now be applied to human health and industry.
The work underscores a growing trend in science: using biomimicry to design materials and technologies that align with the principles of life itself. Instead of imposing control, researchers are learning to cooperate with nature’s logic, harnessing its time-tested strategies for survival, adaptation, and efficiency.
This approach doesn’t just result in functional materials—it produces ones that are more sustainable, more intuitive, and more in tune with the challenges of the modern world.
The Road Ahead: What Comes Next?
While this study marks a powerful proof of concept, many questions remain. How long can the peptides preserve proteins under real-world conditions? Can the method be scaled up for industrial or pharmaceutical use? Are there limitations based on the types of proteins used?
Ulijn and his team are now exploring these questions. They aim to expand the peptide toolkit, optimize formulations, and test a wider range of proteins and environmental conditions. Early collaborations are already forming to investigate commercial applications in vaccine delivery and enzyme preservation.
Moreover, the findings may stimulate interest in the broader field of minimalist materials—those that do more with less. As researchers delve deeper, we may find that the smallest molecules can solve some of the biggest problems.
Conclusion: A Small Leap with Giant Implications
This breakthrough doesn’t come from a billion-dollar lab or futuristic technology. It comes from a deep understanding of nature and a willingness to learn from it. A molecule just three amino acids long, inspired by a microscopic creature’s survival trick, might hold the key to preserving life-saving medicine in the harshest conditions.
That’s the power of good science—not just to understand the world, but to change it. Not by brute force, but by elegant design.
As we face growing global challenges—pandemics, climate change, supply chain disruptions—solutions like this remind us that the answers are often hiding in plain sight, waiting to be noticed in the quiet patterns of nature. Sometimes, all it takes is a closer look and the right question. And when that happens, even the smallest peptide can open the door to a better future.
More information: Adaptive peptide dispersions enable drying-induced biomolecule encapsulation, Nature Materials (2025). DOI: 10.1038/s41563-025-02300-z