In the hushed chambers of the human brain, where thoughts flicker and memories take shape, something can go catastrophically wrong. For children with a condition called Alternating Hemiplegia of Childhood (AHC), this silent storm often begins in infancy. Without warning, their bodies fall into paralysis—sometimes one side, sometimes the other. The episodes are frightening. They can last minutes or stretch into days. They bring muscle spasms, eye movement abnormalities, developmental delays, and terrifying seizures that can strike without mercy.
There is no cure. Until now, treatment has meant managing symptoms, never truly touching the root cause. But a recent breakthrough by a coalition of scientists and advocates may have changed that forever.
A Tiny Injection, A Giant Leap
For the first time, scientists have corrected gene mutations responsible for a neurological disease directly inside the brains of living mice—with just one injection. The results weren’t just molecularly impressive—they were lifesaving.
The study, published in the prestigious journal Cell, was led by a team from The Jackson Laboratory (JAX), the Broad Institute of MIT and Harvard, and the nonprofit RARE Hope. It targeted AHC, a condition so rare that many physicians have never heard of it. Yet its implications go far beyond a single disease. This is not just about saving a few mice, or even a few children. It’s about cracking open the possibility of personalized brain gene editing for a host of genetic disorders long considered beyond hope.
“Five years ago, this would have sounded like science fiction,” said Dr. Markus Terrey, a neuroscientist at JAX and co-leader of the research. “But we did it. We went into the brain of a living organism, fixed the DNA mutation, and watched the corrected cells function normally—for the rest of their lives.”
The Mutation That Unravels a Life
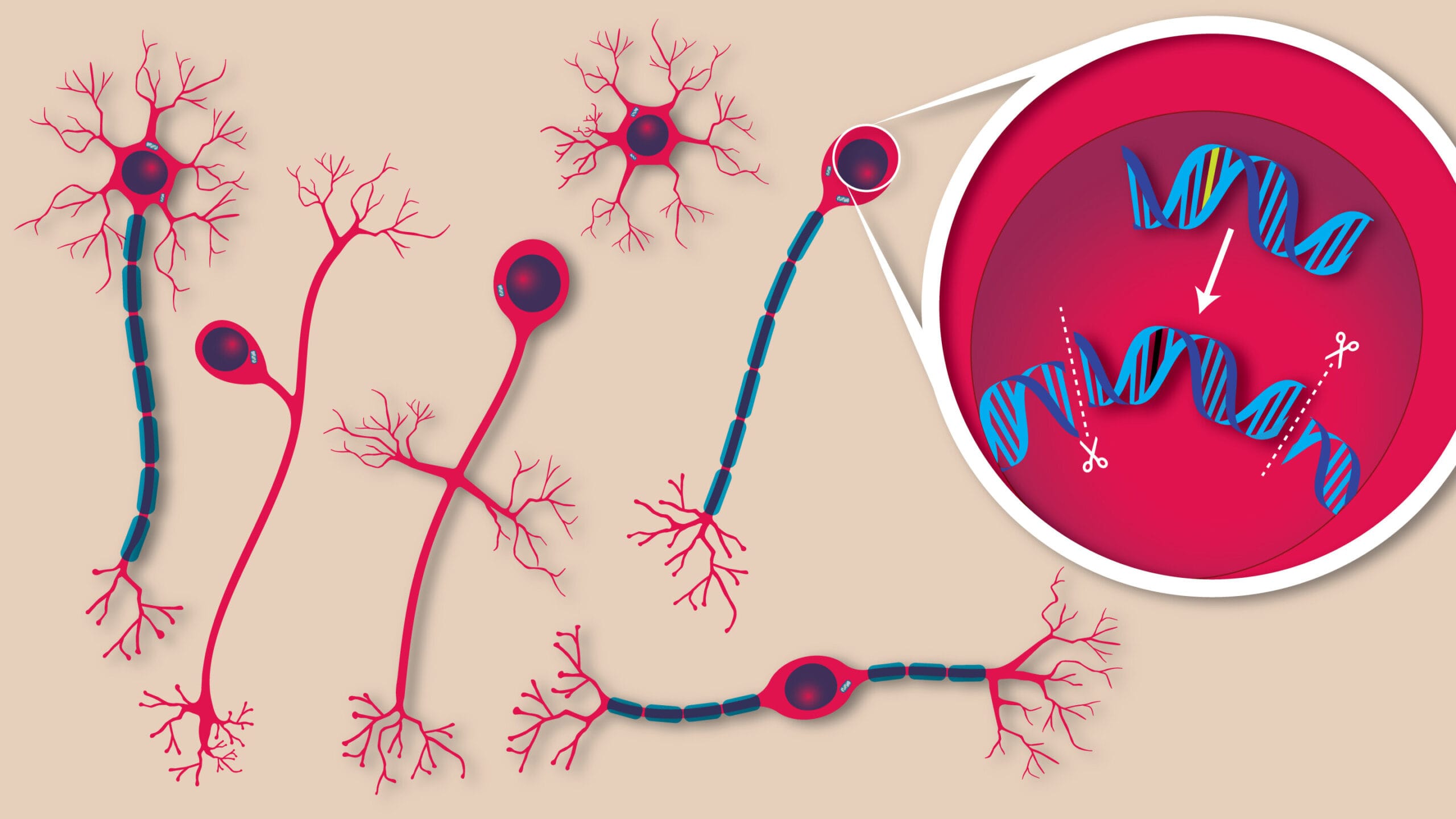
To understand the magnitude of this achievement, one must understand the culprit: a gene called ATP1A3. Within the intricate code of this gene, two particular mutations—D801N and E815K—wreak havoc in the developing brains of children with AHC. These genetic glitches cause disruptions in the proteins that regulate ion flow in brain cells, leading to the disordered neural signals that produce paralysis, seizures, and a kaleidoscope of other symptoms.
It’s like having the wiring in your house short-circuit randomly, shutting down lights, locks, or alarms with no warning or pattern. Worse still, the problem begins before birth, hard-wired into the cells.
Previous attempts to model these mutations in mice resulted in animals that mirrored the human condition: spontaneous paralysis, seizures, premature death. The precision of the models was tragic but necessary—they allowed scientists to test treatments in conditions that faithfully reflected the real disease.
Not a Cure, But a Correction
What sets this study apart isn’t just that it targeted the mutation. It’s how the team did it—and when.
They used a revolutionary form of gene editing called prime editing, developed in 2019 by Dr. David Liu at the Broad Institute, a co-author of this study. Unlike traditional gene therapy, which simply adds a working copy of a gene, prime editing rewrites DNA at its source, like using a word processor to fix a single letter in a sentence without changing the rest.
With prime editing, the scientists were able to correct up to 85% of the faulty gene mutations in neurons—restoring normal protein function, calming seizure activity, and improving motor coordination in the mice. Most remarkably, the mice lived longer. For a disorder where sudden death is a looming threat, even modest extensions in lifespan mark significant progress.
All of this was accomplished through a single injection shortly after birth, using a harmless virus called AAV9 to carry the gene-editing tools past the blood-brain barrier—a biological gatekeeper that usually blocks drugs, viruses, and other therapies from reaching the brain. That delivery success is itself a monumental step forward.
From Fiction to Therapy
For decades, the idea of rewriting genes in the brain sounded fantastical, the stuff of futuristic dreams or dystopian fears. Brains are notoriously difficult to treat, and for good reason: their cells are delicate, their barriers complex, and their functions essential. Editing a single gene in a neuron and having it work safely was once considered nearly impossible.
That’s why the implications of this work are so profound.
“This is one of the most exciting examples of therapeutic gene editing to come from our team,” said Dr. Liu. “It opens the door to one day repairing the genetic causes of neurological disorders once thought untreatable.”
What makes this moment even more powerful is that it wasn’t driven by scientists alone. It was shaped, in part, by a mother.
Driven by Love and Urgency
Nina Frost is the founder and president of RARE Hope, a nonprofit born from a mother’s desperation to help her daughter, Annabel, who has AHC. Frustrated by the lack of answers, Nina did what few parents ever imagine—they helped build a scientific movement. RARE Hope connected leading researchers with families, patients, and advocates, and helped make sure the science was not only rigorous but meaningful.
RARE Hope’s influence wasn’t just symbolic. It helped shape the study’s experimental design, data interpretation, and research priorities. Patient voices didn’t just whisper in the background; they sang through the science.
“Seeing data that showed not just molecular correction, but functional rescue in mouse behavior—it was an incredibly exciting moment,” Frost said. “Up until now, we didn’t know if this was a disease that could be rescued postnatally.”
Beyond AHC: A Gateway to More
While AHC itself is vanishingly rare—affecting perhaps one in a million—the broader implications of this work are enormous. Around 80% of rare diseases are genetic. Most have no treatment, and many affect the nervous system. The idea that scientists can now go directly into the brain and correct the DNA before—or even after—symptoms appear could rewrite the future for countless patients.
Even more thrilling is that the team didn’t just prove the concept with one variant. The published study included results from five distinct mutations, giving scientists hope that the approach can be expanded across multiple diseases.
“We’re not just seeing one success story,” said Terrey. “If we can do it for one mutation, we can reasonably assume we can do it for others. We now have a roadmap.”
The Next Frontier: Reversing Time
The study’s initial treatment was given shortly after birth, before severe symptoms took hold. That timing was deliberate—it allowed the gene-editing tools to reach neurons before irreversible damage occurred. But for many patients, a diagnosis doesn’t come early. Parents often spend months or years searching for answers as symptoms worsen.
Now, the scientists are working to answer the next big question: Can this treatment reverse the disease after it starts?
“We haven’t necessarily reversed the disease,” admitted Dr. Cathleen Lutz, vice president of the Rare Disease Translational Center and co-leader of the study. “But we’ve shown we can reduce symptoms if we act early. The next big test is to treat mice that already show signs—seizures, dystonia, paralysis—and see if we can still make a difference.”
If they succeed, it would mark a paradigm shift: not just prevention, but repair.
The Ethical Horizon
The ability to edit genes in the brain also brings a wave of ethical questions. How far should we go? What conditions should be treated? How do we ensure safety and fairness? While this study focused on life-threatening mutations with clear neurological consequences, future applications may blur the lines between therapy and enhancement.
Still, for families staring down the barrel of rare, devastating conditions, the science offers more than answers—it offers hope.
“This isn’t just a paper,” said Frost. “It’s a signal to families that they have not been forgotten. That their lives matter. That science can catch up to their suffering.”
Rewriting Tomorrow
In the end, this study is about more than fixing letters in DNA. It’s about rewriting stories—ones that were once guaranteed to end in loss, but now shimmer with new possibilities. It’s about children who may someday live without fear of collapsing muscles or silent seizures. It’s about science that doesn’t just seek to understand the brain but dares to change its destiny.
We are living in a new era—one where the brain, the final frontier of medicine, is finally becoming accessible. And with every corrected gene, every rescued mouse, and every emboldened family, the line between what is and what could be grows ever thinner.
The future, it seems, is editable.
Reference: In vivo prime editing rescues alternating hemiplegia of childhood in mice, Cell (2025). DOI: 10.1016/j.cell.2025.06.038