Deep within the symphony of the immune system exists a quiet, sophisticated group of cells that rarely make headlines but are essential to life. They don’t kill viruses, nor do they charge into infections with the brute force of inflammation. Instead, they calm, orchestrate, and rebuild. These are Regulatory T cells, or Treg cells, and though their name might sound technical, their role is anything but cold and mechanical. They are the immune system’s peacekeepers and architects—balancing defense with restraint, and destruction with renewal.
While most immune cells are trained to fight, Treg cells are designed to protect us from ourselves. Without them, the immune system can turn against its host, mistaking healthy tissue for a threat. This is the root of autoimmune diseases, and it’s here that Treg cells emerge not as passive monitors but as decisive regulators, suppressing the misfires before they become catastrophic.
Yet their mission doesn’t stop at preventing harm. Treg cells are also deeply involved in wound healing and tissue regeneration, acting like molecular repairmen who rush in after the chaos of infection or injury to rebuild what was broken. They partner with other immune cells, as well as the body’s own stem cells, not just to mend damage but to restore function, resilience, and balance.
The Mystery of Transformation
But there’s a mystery: how do these cells, forged in the crucible of immune training, learn to become tissue healers? Treg cells don’t begin their lives ready to repair wounds. They have to transform, adapting themselves to specific tissues—be it the lungs, liver, skin, or intestines—each with its own unique challenges and healing needs. Scientists call this transformation process the development of tissue-Treg cells.
It’s a biological alchemy that has intrigued immunologists for years, not just for its elegance but for its therapeutic potential. If we could understand how Treg cells become tissue healers, we might learn how to harness or even replicate this ability to treat diseases—from chronic inflammation to autoimmune disorders to organ damage.
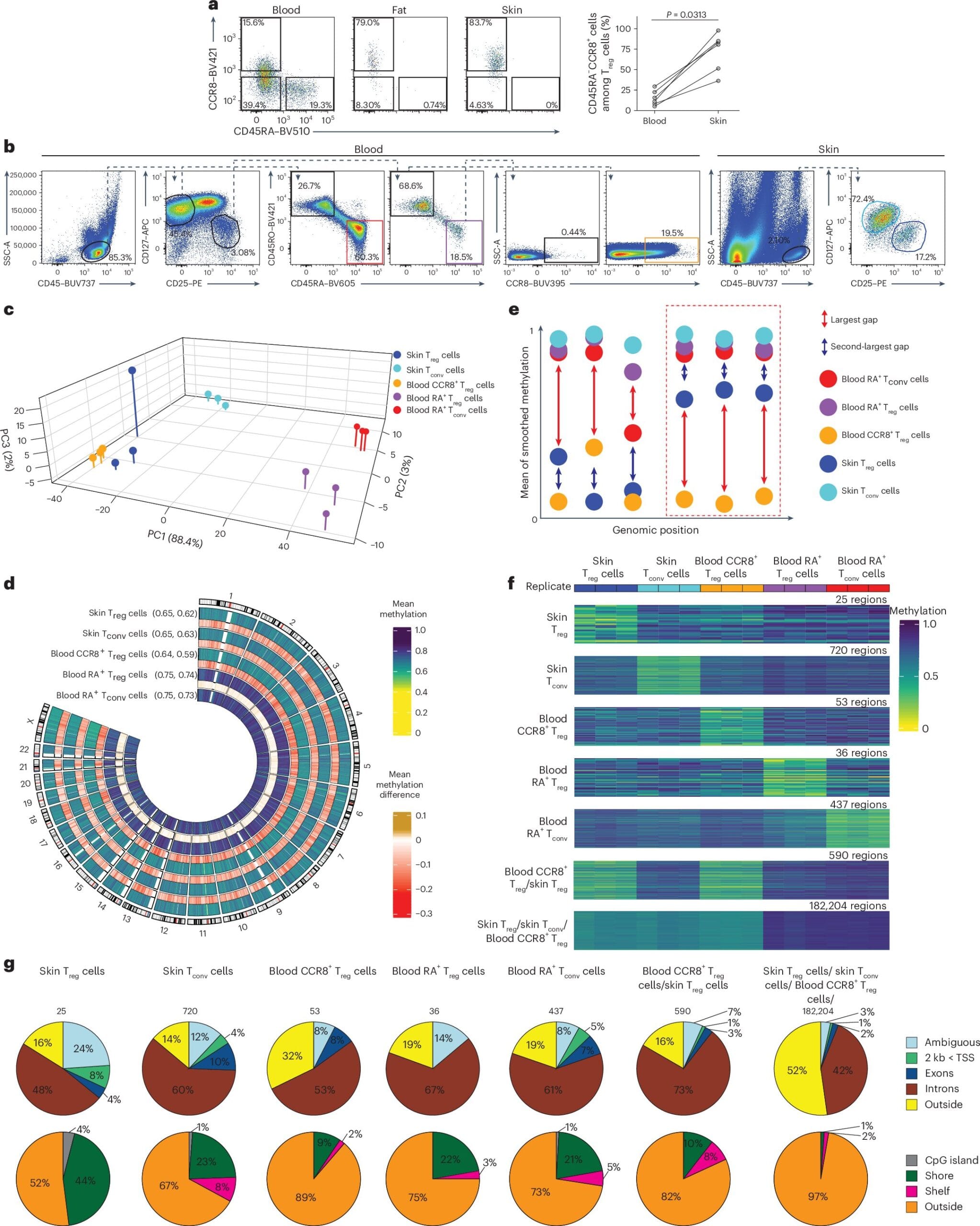
Now, thanks to a groundbreaking study published in Nature Immunology, we are closer than ever to understanding this transformation. Scientists from the LIT Division of Immunology, in collaboration with the University Medical Center Mainz and the German Cancer Research Center, have unraveled the intricate epigenetic changes that enable Treg cells to become tissue-specific repair cells in humans.
Beyond the Code: The Power of Epigenetics
To grasp this discovery, it’s essential to understand what epigenetics is and why it matters. Every cell in the human body contains the same DNA—our genetic blueprint—but not all genes are active in every cell. The difference between a skin cell and a liver cell, or between a normal T cell and a tissue-Treg cell, isn’t the genes themselves—it’s which ones are turned on or off.
Epigenetics refers to this system of genetic “switches”—a molecular control panel that tells each cell which genes to activate and which to silence. And unlike DNA mutations, which change the genetic code permanently, epigenetic changes are reversible and dynamic, allowing cells to adapt to new environments or roles.
In this study, the scientists zeroed in on a specific type of epigenetic change called DNA methylation. This involves tiny molecules, known as methyl groups, attaching themselves to certain spots on the DNA—especially at areas called CpG sites. Think of these as notes scribbled in the margins of your genome—hints that tell the cell how to interpret the underlying text.
“When a gene near a CpG site is marked with a methyl group, it’s typically silenced. But if it’s left unmarked, the gene can be switched on,” explains Tamara Kaufmann, one of the study’s authors. This is not random—it’s a finely tuned system of cellular memory, crucial to the identity and function of each cell.
The Fingerprint of a Healer
By mapping these methylation patterns across millions of CpG sites, the research team uncovered something extraordinary: a genetic fingerprint unique to human tissue-Treg cells. These patterns weren’t just scattered changes—they told a story, a narrative of transformation, from an ordinary immune regulator to a specialized tissue repair cell.
This fingerprint is more than a signature. It acts as a diagnostic tool, allowing scientists to identify tissue-Treg cells not just in damaged tissues, but even while they’re still circulating in the blood. It’s like spotting an architect in a crowd before the blueprints are even drawn.
“Our findings revealed how these tissue-Treg cells carry epigenetic markers that define their healing function,” says Prof. Delacher, one of the leaders of the study. “They’re biologically distinct and specially equipped for regeneration.”
But there was another surprise hidden in the genome.
The Ghosts in Our Genes: Jumping Genes Come Alive
During their analysis, the team also discovered that many of the changes occurred in transposable elements—better known as jumping genes. These are ancient stretches of DNA that, once upon a time, could move around the genome. For decades, scientists believed they were relics—genetic fossils left behind by evolution with no real function.
But that view is changing. Recent studies suggest that jumping genes may serve as regulatory hubs, influencing how nearby genes behave. In this study, they appeared to be active participants in the reprogramming of Treg cells, shaping their identity as tissue healers.
“These elements aren’t just junk,” notes Prof. Feuerer, another senior author. “They’re part of a complex language that the genome uses to control itself. And in tissue-Treg cells, they seem to be writing a very specific message.”
A Future of Tailored Healing
The implications of these discoveries go far beyond the lab. With this new understanding of how Treg cells adapt to tissue environments, scientists can begin to design therapies that mimic or support this natural process. Imagine boosting tissue regeneration after a heart attack, calming chronic inflammation in diseases like Crohn’s or lupus, or restoring balance in autoimmune conditions like type 1 diabetes—all by guiding the behavior of the body’s own cells.
It’s not science fiction. It’s a vision increasingly grounded in the hard evidence of molecular biology.
What’s especially powerful is that these therapies could be precision-targeted. Because the epigenetic fingerprints are so specific, treatments could be tailored not just to the disease, but to the individual tissue—whether it’s the lung, skin, brain, or gut. And because epigenetic changes are reversible, we may one day learn how to retrain Treg cells in real-time, adjusting their behavior as diseases progress or heal.
The Quiet Revolution Inside Us
The story of regulatory T cells is a story of quiet intelligence. Unlike the immune system’s warriors that fight loudly and visibly, Treg cells operate in whispers, fine-tuning the balance between aggression and peace. They are not there to win battles—they are there to restore order.
And now, for the first time, we are beginning to read their code.
With every CpG site mapped, with every methylation mark uncovered, scientists are pulling back the veil on one of biology’s most elegant systems of control. And behind that veil lies not only the promise of better health but a deeper understanding of how the body heals, adapts, and survives.
The discovery that our cells carry not just blueprints but also instructions for how to interpret them, speaks to the remarkable complexity of life. Treg cells are not passive responders—they are decision-makers, able to sense, adapt, and transform in ways we are only beginning to understand.
In them, we see not just immune regulators—but the future of medicine.
Reference: Niklas Beumer et al, DNA hypomethylation traits define human regulatory T cells in cutaneous tissue and identify their blood recirculating counterparts, Nature Immunology (2025). DOI: 10.1038/s41590-025-02210-x