It’s easy to overlook the invisible. Every day, we walk under an expansive sky, rarely giving thought to the ocean of air that surrounds us. We breathe in. We exhale. We talk about the weather. But few pause to appreciate the intricate, life-preserving marvel that is Earth’s atmosphere. It’s not just a blanket of gas—it’s a guardian, a life-giver, and the unseen architecture of every living moment on this planet.
Imagine, for a moment, that Earth’s atmosphere vanished. In seconds, chaos would erupt. Water would boil away into space, ultraviolet radiation would fry unprotected skin, and life—as we know it—would cease to exist. The truth is simple yet staggering: without the atmosphere, Earth would be a cold, sterile rock floating through a hostile void.
But what exactly makes this mix of gases so essential to life? How does it cradle the biosphere, shape evolution, and stabilize the chaos of the cosmos? The story of Earth’s atmosphere is not just a scientific tale—it’s a planetary epic, a testament to nature’s finely tuned balance.
The Birth of an Atmosphere: A Rocky Start
When Earth was born some 4.5 billion years ago, it wasn’t the serene blue world we know today. It was a hellish inferno, constantly battered by meteorites and volcanically explosive. Its first atmosphere was likely composed of hydrogen and helium—light gases captured from the solar nebula. But this primitive shroud was fleeting. Solar winds stripped it away quickly, leaving Earth temporarily naked to the void.
Then, something extraordinary happened. Volcanoes, bursting with energy, began to pour out gases like water vapor, carbon dioxide, nitrogen, and sulfur. This second atmosphere—harsh and toxic to us now—set the stage for transformation. There was no oxygen, no ozone layer, and certainly no life. But in the scorching brew of volcanic heat and oceanic turbulence, complex chemistry began.
Among the bubbling primordial soups and hydrothermal vents, life found its first foothold. Microorganisms—simple, microscopic, yet astonishingly adaptable—emerged. And one group, cyanobacteria, would alter the fate of the planet forever.
The Oxygen Revolution: When Life Transformed the Sky
Cyanobacteria, also known as blue-green algae, are credited with pulling off one of the most audacious acts in planetary history: they started producing oxygen through photosynthesis. Slowly, molecule by molecule, they released this new gas into the atmosphere. It took hundreds of millions of years, but eventually, oxygen began to accumulate.
At first, oxygen was a threat to most existing anaerobic life. It was a reactive element—causing what could be described as a global ecological crisis for early microbes. But this so-called “Great Oxidation Event” roughly 2.4 billion years ago was the dawn of a new world. With oxygen, the atmosphere gained a new dimension. Energy production in cells became more efficient, multicellular organisms began to evolve, and the seeds of complex life were sown.
As oxygen levels rose, another miracle occurred high above the ground. Molecules of O₂, bombarded by sunlight, began to split and recombine into O₃—ozone. This fragile, bluish gas formed a thin layer in the stratosphere that absorbed deadly ultraviolet radiation. With this protective veil in place, life was finally free to crawl out of the oceans and onto dry land.
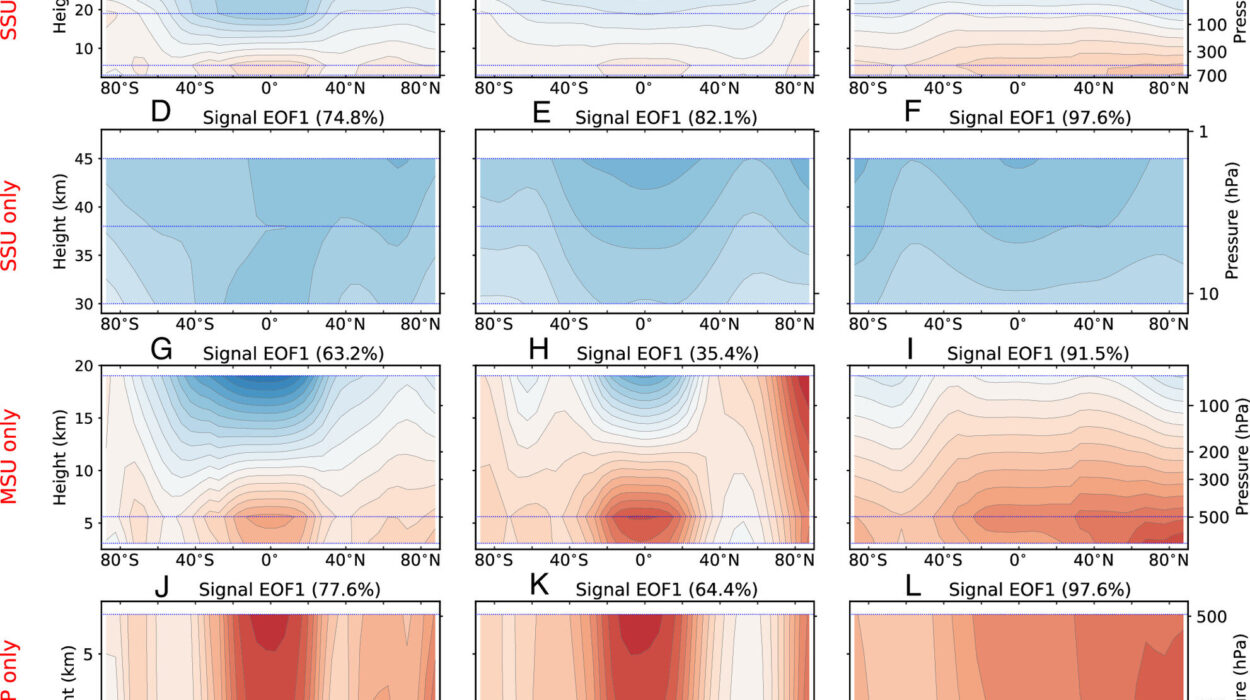
The Atmosphere’s Five-Layered Symphony
To the untrained eye, the atmosphere might seem like a single, undifferentiated shell. But in truth, it is a masterpiece of structure and function, divided into five distinct layers, each with its own role in protecting and nurturing life.
At the surface lies the troposphere, the lowest layer where weather happens, clouds form, and breathable air exists. It stretches up about 8 to 15 kilometers, thinning as it rises. Almost all of life’s drama—from hurricanes to gentle breezes—unfolds here.
Above that is the stratosphere, home to the crucial ozone layer. Temperatures rise with altitude here, a counterintuitive trait driven by ozone absorbing solar energy. This stable layer is also where high-altitude aircraft and weather balloons travel.
Next comes the mesosphere, the coldest layer, where temperatures can plummet below -90°C. Here, meteors burn up in streaks of fiery light, protecting the surface from bombardment.
The thermosphere follows—a region where solar activity causes dramatic temperature spikes. It’s where the auroras shimmer and satellites orbit, brushing against the edge of space.
Finally, the exosphere fades into the interplanetary void. This outermost layer is more a transition than a boundary, where atoms and molecules escape Earth’s grip and drift into infinity.
Together, these layers form a dynamic system that moderates temperature, filters radiation, supports weather cycles, and shelters the biological world in a cocoon of stability.
A Breath of Life: The Chemistry of Survival
Every inhale is a silent miracle. The air that fills your lungs is a mix of gases, precisely balanced for life. Roughly 78% nitrogen, 21% oxygen, and 1% other gases—including argon, carbon dioxide, neon, and trace elements—make up our atmosphere.
Nitrogen, though seemingly inert, plays a vital role in the biogeochemical cycling of nutrients. Plants need it to build proteins and DNA, but they can’t use atmospheric nitrogen directly. Enter the nitrogen cycle: a complex interaction involving bacteria, soil, and plant roots that convert nitrogen into usable forms, linking the atmosphere to the food chain.
Oxygen, of course, is indispensable for respiration in most life forms. It allows cells to efficiently convert food into energy. Too little, and life gasps. Too much, and it can become toxic. The balance we enjoy today wasn’t always guaranteed—it’s the result of eons of biological regulation.
Then there’s carbon dioxide. Often vilified today because of its role in climate change, CO₂ is nonetheless essential. Plants absorb it during photosynthesis, converting sunlight into sugar and releasing oxygen in return. It’s the cornerstone of the carbon cycle, tying the atmosphere, oceans, and biosphere into a single interconnected system.
Even the trace gases have stories. Ozone blocks ultraviolet rays. Water vapor controls temperature and drives weather. Methane, though present in small amounts, is a powerful greenhouse gas, contributing to Earth’s warmth.
Every molecule plays a role in the grand atmospheric ballet. Without this precise mix, the chemistry of life would unravel.
The Climate Regulator: Guarding Earth’s Temperature
Space is cold—brutally, unimaginably cold. But Earth remains warm and cozy thanks to the atmosphere’s insulating properties. The atmosphere traps heat from the sun through what’s known as the greenhouse effect. It works like a thermal blanket: solar radiation penetrates the atmosphere and warms the surface. Earth then radiates this heat outward as infrared energy, but certain gases—chiefly carbon dioxide, methane, and water vapor—trap some of that heat and reflect it back, keeping the planet habitable.
Without this natural greenhouse effect, Earth’s average temperature would be -18°C, a frozen wasteland. Instead, we enjoy a balmy average of about 15°C.
But like any delicate system, balance is crucial. Human activities—burning fossil fuels, deforestation, industrial agriculture—have upset this equilibrium. Carbon dioxide levels have soared since the Industrial Revolution, driving temperatures upward and disrupting global climate systems. While this is a modern crisis, it’s rooted in the same atmospheric mechanisms that have always sustained life.
Weather and the Water Cycle: The Lifeblood of Ecosystems
Every drop of rain, gust of wind, or rolling thunderstorm is powered by the atmosphere. It’s not just a passive envelope—it’s an engine, a circulatory system for the planet.
The sun heats Earth unevenly, warming equatorial regions more than the poles. This creates pressure differences that drive wind, moving air masses around the globe. The rotation of the Earth adds complexity, spawning trade winds, jet streams, and cyclones.
Meanwhile, water evaporates from oceans and lakes, rises into the atmosphere, condenses into clouds, and falls as rain or snow. This hydrological cycle, sustained entirely by atmospheric dynamics, nourishes every ecosystem on Earth. It delivers water to forests, replenishes rivers, irrigates crops, and sustains wildlife.
The atmosphere is also nature’s cleanup crew. It distributes nutrients like dust and pollen. It filters out pollutants. It even disperses seeds and spores, helping plants colonize new terrain. Without atmospheric motion, life would stagnate, stranded in ecological isolation.
A Shield Against the Cosmos
Earth’s atmosphere is not just a cradle—it’s also a shield. Space is a violent place, filled with hazards: solar flares, cosmic rays, meteorites, and ultraviolet radiation. The atmosphere fends them off with silent heroism.
The ozone layer filters out most of the sun’s harmful UV-B and UV-C radiation. Without it, skin cancers would skyrocket, crops would wither, and DNA would unravel in a cellular apocalypse.
Meteorites, which bombard our planet daily, usually burn up in the mesosphere, thanks to friction with atmospheric particles. What might be planet-killing projectiles end up as harmless shooting stars.
Meanwhile, high in the thermosphere, solar particles interacting with Earth’s magnetic field create auroras—glorious light shows that are the visible tip of a protective spear. The atmosphere dissipates this energy before it can reach the surface.
In short, Earth’s atmosphere is a nearly invisible force field—a planetary armor forged over billions of years.
A Fragile Balance: The Human Responsibility
Though powerful, Earth’s atmosphere is not indestructible. Human actions are stretching it to its limits. Air pollution darkens skies and chokes cities. Greenhouse gases intensify warming. Ozone-depleting chemicals once threatened the stratosphere. Deforestation and ocean warming alter atmospheric feedback loops.
But there’s hope. The ozone crisis of the 1980s led to the Montreal Protocol, a landmark international agreement that phased out harmful chemicals. The ozone layer has been recovering, showing that global cooperation and science-driven policy can make a difference.
Now, as climate change looms as the defining issue of our time, the atmosphere again calls out for stewardship. Reducing emissions, protecting forests, and embracing sustainable energy are not just political issues—they are existential ones.
Looking Beyond Earth: Atmospheric Lessons from Other Worlds
To appreciate the miracle of Earth’s atmosphere, we need only look at our neighbors.
Venus, shrouded in thick carbon dioxide, has a runaway greenhouse effect. Surface temperatures soar above 460°C, hot enough to melt lead. Mars, by contrast, has an atmosphere too thin to trap heat, leaving it barren and frigid, with no protection from solar radiation.
These planetary contrasts underscore how rare and precious Earth’s atmosphere is. It didn’t arise by accident. It was shaped by geological upheaval, biological innovation, and the delicate balance of cosmic forces.
Conclusion: The Air We Breathe, The Life We Live
Earth’s atmosphere is more than air. It’s the breath in our lungs, the warmth on our skin, the water in our veins. It is at once a memory of Earth’s fiery birth and the ongoing poetry of life’s persistence.
Invisible yet indispensable, the atmosphere is the most eloquent silence we will ever know. It whispers across time, carrying the secrets of evolution, the footprints of ancient volcanoes, and the song of the wind. It speaks not only of what is, but of what could be—if we listen, if we care, and if we protect this thin blue veil that lets life flourish on a lonely rock in a vast, indifferent universe.