In the heart of every living organism lies an orchestra of cellular activity, each cell a performer playing its role with exquisite precision. But like all performances, life’s symphony eventually winds down—and often not peacefully. While much attention has been devoted to how cells live, divide, and die in orderly fashion, a lesser-known but far more violent end lurks in the shadows: necrosis. Once thought to be simply a chaotic endpoint—a messy footnote to an otherwise well-scripted biological story—necrosis is now stepping into the spotlight, and scientists believe it may hold the key to extending human life, combating age-related disease, and even enabling deep space travel.
This revolutionary idea is at the heart of a new study published in Oncogene by researchers from University College London (UCL), the European Space Agency (ESA), and the biotechnology company LinkGevity. In this landmark paper, an international team of scientists and clinicians challenges the traditional view of necrosis as merely a symptom of disease and aging. Instead, they propose something much more provocative: that necrosis is not just a consequence, but a driving mechanism of human degeneration—and a tantalizing opportunity for intervention.
A Death Worth Understanding
“People don’t like talking about death, especially not at the cellular level,” says Dr. Keith Siew of UCL’s Centre for Kidney & Bladder Health, co-author of the study. “But if we really want to understand aging and disease, we need to understand how and why cells die the way they do. Because when cells die, it’s not just about that cell—it’s about everything that happens next.”
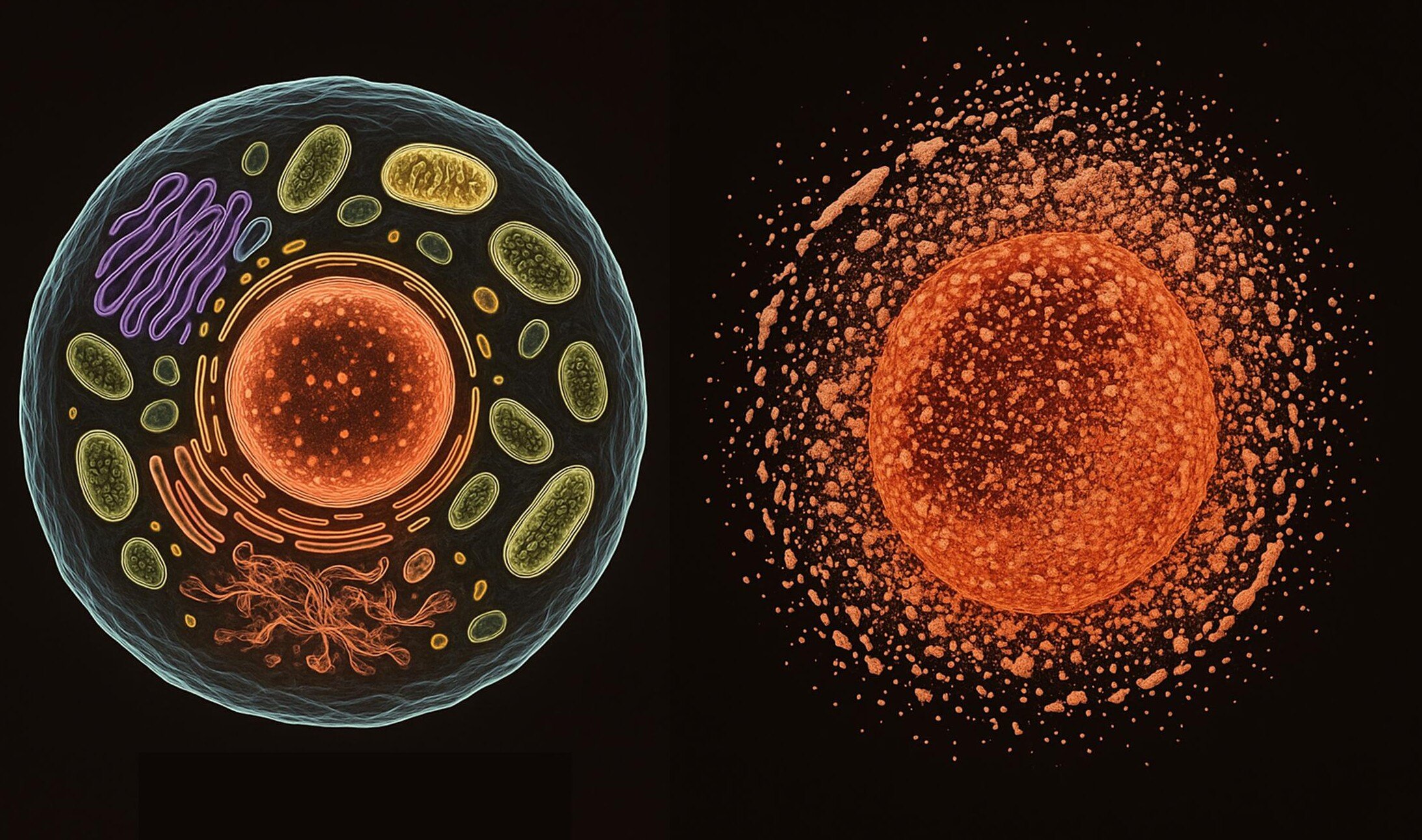
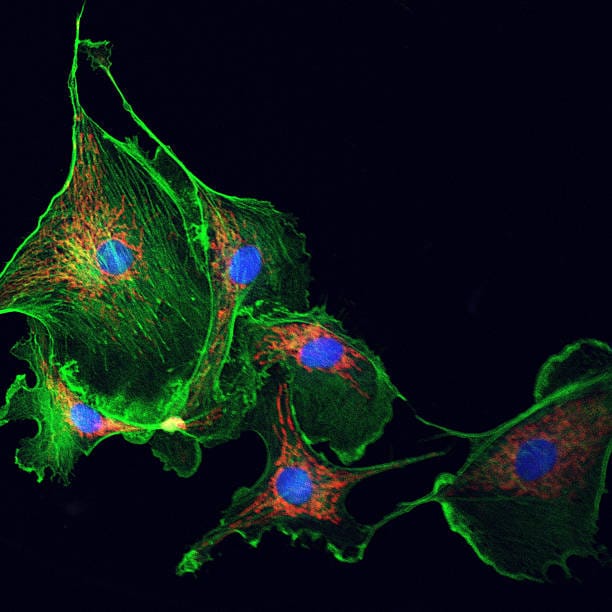
Cells can die in many ways. Some methods, like apoptosis, are well-behaved and programmed, an elegant suicide that allows the body to recycle and replace cellular material without fuss. But necrosis is different. It’s raw, catastrophic, and unplanned. Triggered by infection, injury, toxins, or extreme stress, necrosis is less like a peaceful farewell and more like an explosion. It causes the cell to rupture, spilling its contents—including inflammatory molecules—into the surrounding tissue. This not only disrupts neighboring cells but sparks a damaging chain reaction, much like a fire spreading from house to house.
And that, scientists now argue, may be one of the core engines behind aging itself.
The Calcium Catastrophe
At the molecular heart of necrosis lies a tiny but mighty element: calcium. Inside healthy cells, calcium levels are kept incredibly low—up to 100,000 times lower than outside the cell. This steep gradient allows calcium ions to act as messengers, signaling when a cell should grow, divide, repair, or rest. But when stress hits hard enough to break the barrier, calcium floods in, short-circuiting the cell’s internal machinery.
Imagine a precision clock suddenly deluged by water: its gears grind, its circuits spark, and within moments, order collapses into chaos. The cell begins to swell, its membrane ruptures, and its contents spill into the extracellular environment. Unlike the tidy dissolution seen in programmed death, necrosis is loud, inflammatory, and deeply disruptive.
“Necrosis has been hiding in plain sight,” says Dr. Carina Kern, the study’s lead author and CEO of LinkGevity, a biotech firm working at the nexus of longevity science and space health. “It’s often viewed as the last act in a disease process. But what we’re seeing is that necrosis may actually be the opening act—setting the stage for further damage and degeneration. If we can pause or prevent it, we might be able to completely reshape how we treat age-related conditions.”
Aging in Reverse?
The study proposes that necrosis doesn’t just accompany aging—it accelerates it. As we age, tissues become more vulnerable to the kinds of stress that trigger necrosis: inflammation, oxidative stress, mitochondrial dysfunction, and immune dysregulation. Once necrosis begins, it can spark a cycle of injury and impaired healing. Damaged tissues become fibrotic and inflamed, while regenerative processes grind to a halt. The effect snowballs, leading to conditions such as heart disease, kidney failure, neurodegeneration, and even certain cancers.
Dr. Kern describes necrosis as the “missing link” that connects seemingly unrelated diseases. “If you look at Alzheimer’s, kidney disease, heart failure—they all involve cycles of cell damage and poor regeneration. And in all of them, necrosis plays a key role in kicking off those cycles,” she explains. “Interrupting necrosis, even temporarily, could give the body a chance to heal before the damage becomes irreversible.”
This is more than just theoretical. In animal models, researchers have already shown that blocking specific pathways associated with necrotic cell death can reduce tissue damage and improve outcomes. New experimental compounds are being developed to modulate necrosis, with some already in early-stage trials for degenerative diseases. The next frontier, say researchers, is targeting necrosis as a systemic intervention—not just in a single disease, but across the aging process itself.
The Kidney: Necrosis’s Silent Victim
Nowhere is the impact of necrosis more evident—and more underappreciated—than in the kidneys. These delicate filtration systems are among the most vulnerable organs in the body, subjected to a constant stream of metabolic waste, immune activity, and environmental toxins. According to Dr. Siew, by age 75, nearly half of people show signs of chronic kidney disease, even if they’re unaware of it.
“Kidney failure isn’t caused by one thing,” he explains. “It’s caused by a thousand small insults—poor blood flow, inflammation, oxidative stress—and all of them converge on necrosis. The kidney cells get overwhelmed, and once necrosis starts, it feeds on itself. You get fibrosis, further damage, and eventually you reach a tipping point where dialysis or transplant is the only option.”
But if necrosis could be intercepted early—before that spiral begins—then doctors might be able to prevent or delay kidney failure altogether. “We can’t stop every stressor,” says Siew. “But if we can stop necrosis, we may not have to.”
Spaceflight and the Aging Paradox
As humanity sets its sights on Mars and beyond, the challenges of long-term space travel are taking center stage. One of the most concerning findings from recent space missions is that astronauts experience accelerated aging: bone loss, muscle wasting, immune dysfunction, and, notably, kidney decline.
In 2024, Dr. Siew co-authored a paper showing that the kidney may be the single greatest bottleneck to extended human spaceflight. In microgravity, calcium homeostasis is disrupted, and the stresses on kidney function increase dramatically. Cosmic radiation compounds the problem, causing DNA damage that further accelerates necrosis.
Professor Damian Bailey of the University of South Wales and Chair of ESA’s Life Sciences Working Group says the implications are profound. “If we don’t solve the necrosis problem in space, we may never get humans to Mars safely,” he says. “But the flip side is just as exciting—if we solve it in space, those solutions will transform health here on Earth.”
Dr. Kern agrees. “Necrosis is the great equalizer. It affects astronauts in space, patients in intensive care, and older adults in everyday life. It’s the final common pathway in so many chronic conditions. If we can block it, even temporarily, we may give the body a fighting chance to repair, regenerate, and thrive.”
Toward a New Era of Regeneration
Perhaps the most tantalizing promise of necrosis research lies in its potential to unlock regeneration. Normally, when a cell dies in an orderly way, nearby stem cells step in to replace it. But when necrosis occurs, the environment becomes toxic—fibrosis replaces functional tissue, inflammation suppresses repair, and cells that might have divided and healed the wound instead lie dormant or die themselves.
“If we can keep necrosis at bay, even briefly, we might allow the body’s natural healing systems to kick in,” says Dr. Kern. “We’re not talking about immortality. We’re talking about giving tissues the conditions they need to function better for longer.”
The paper, which includes contributions from institutions such as Harvard Medical School, Mayo Clinic, and the MRC Laboratory of Molecular Biology, is not a call to hype but a call to action. The authors argue that necrosis has been sidelined in the rush to understand apoptosis and other better-known cellular mechanisms—but that is about to change.
“This is a missing piece of the puzzle,” says Professor Bailey. “We’ve spent decades trying to slow aging from the outside in. Necrosis lets us go from the inside out.”
What Comes Next?
Already, biotech startups are exploring necrosis-targeting drugs, with some focusing on calcium signaling, others on mitochondrial stability, and still others on immunomodulation to clean up the damage after cell death. The goal is not to eliminate necrosis completely—it is, after all, a natural response to overwhelming stress—but to modulate it, to tip the balance back toward repair rather than collapse.
Dr. Kern’s company, LinkGevity, is working on early-stage compounds and hopes to launch clinical trials in the coming years. Meanwhile, space agencies are integrating necrosis research into their astronaut health protocols, seeking ways to protect kidneys, hearts, and bones on long missions.
If this research succeeds, it may mark a fundamental shift—not only in how we treat diseases like Alzheimer’s or kidney failure, but in how we think about aging, vitality, and even our place in the cosmos.
“We’re standing at the edge of a new frontier,” says Dr. Siew. “And the door may open not with a bang, but with a cell choosing not to die.”
Reference: Carina Kern, et al. Necrosis as a fundamental driver of loss of resilience and biological decline: What if we could intervene?’, Oncogene (2025). DOI: 10.1038/s41388-025-03431-y