Life on Earth has always been the great alchemist of the cosmos—capable of transforming simple molecules into conscious beings. From the single-celled organisms that first wiggled through primordial oceans to the neurons that now contemplate the nature of the universe, all life shares one striking feature: the ability to reproduce. That ability, however, has always been chained to the exquisite complexity of biochemistry—an intricately coordinated ballet of DNA, RNA, proteins, lipids, and enzymes.
But what if reproduction—the biological bedrock of life—could arise in a world entirely devoid of biochemistry? Could nature’s most vital property emerge in systems where there are no genes, no membranes, no metabolism? In a groundbreaking study published in Proceedings of the National Academy of Sciences (PNAS), researchers from Harvard University have built a striking answer: Yes, it can.
Their work describes a stunning achievement—a synthetic, abiotic system that mimics cellular self-reproduction, forming life-like vesicles that grow and divide, not by DNA instruction or enzymatic guidance, but through pure, designed chemistry. This is not just an incremental step in synthetic biology—it’s a fundamental leap into the unknown.
Rethinking the Origins of Reproduction
To understand the weight of this discovery, we must return to a core axiom of biology. In 1858, the German pathologist Rudolf Virchow famously declared, “Omnis cellula e cellula”—every cell comes from a pre-existing cell. For over a century, this maxim has shaped our understanding of life’s continuity. From microscopic bacteria to blue whales, life reproduces by passing down its cellular architecture and biochemical instructions.
At the cellular level, reproduction is a symphony of interactions: DNA replication, lipid bilayer formation, protein synthesis, metabolic coordination. Even the “simplest” single-celled organisms depend on tens of thousands of precisely choreographed reactions. This complexity has led biologists and chemists to ask: Is life’s ability to self-replicate inextricably bound to biochemistry? Or could there be an alternative—a form of self-replication born from non-biological principles?
Previous studies flirted with this idea. Some showed that synthetic micelles or vesicles could grow via polymerization-induced self-assembly (PISA), and others hinted at structural mimicry of cell division. But until now, no system had demonstrated autonomous, sustained self-reproduction in a strictly non-biological medium.
The Harvard team decided to change that.
Designing a Biochemistry-Free Reproduction Engine
To tackle this grand challenge, the researchers crafted a chemical environment utterly devoid of life’s traditional machinery. Their goal was to construct a self-replicating system from scratch—no enzymes, no nucleic acids, no cell membranes. Just chemicals, light, and a clever design.
Their experimental setup resembled something deceptively simple: a one-pot chemical reactor. Into a glass vial went a specially selected hydrophilic polymer that carried a hydrophobic chain transfer agent (CTA) molecule on one end. This combination was essential for kickstarting the polymerization process. Next, they added a monomer—the building block of new polymers—and a photocatalyst, which would activate the reaction under green light. All of this was submerged in water and sealed under a nitrogen atmosphere to exclude oxygen and mimic early Earth’s reducing conditions.
The entire concoction was then exposed to 530-nanometer green LED light at a temperature of 33°C—a relatively mild environment. What followed was both visually subtle and conceptually profound.
From Molecules to Mimics of Life
As green light bathed the solution, it triggered a process known as photo-Reversible Addition-Fragmentation Chain Transfer (RAFT) polymerization—a method that allows for precise control over how polymers grow. The light activated the photocatalyst, which initiated the controlled addition of monomer units to the polymer chains.
Over the course of 90 minutes, something extraordinary began to happen. Amphiphilic block copolymers—molecules with both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions—began to form. Much like lipids in a biological cell membrane, these amphiphiles spontaneously self-assembled into vesicle-like structures: tiny, hollow spheres that resemble the simplest kind of cell.
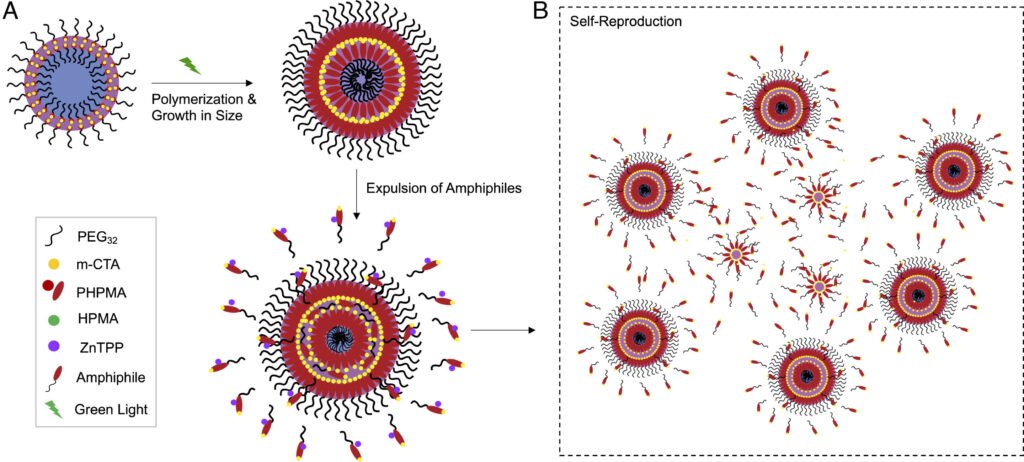
But the resemblance didn’t stop at structure. These synthetic vesicles displayed a startling behavior—they grew, and then, like biological cells, they divided. Over time, they began to release tiny, polymeric “spores”—daughter vesicles that initiated further rounds of growth and division, forming new generations that inherited properties from their predecessors.
For the first time, researchers had witnessed what amounts to abiotic cellular self-reproduction.
What Makes This System Different?
The importance of this experiment lies not just in the behavior observed, but in how it arose. Many previous studies on vesicle growth relied on biochemical components—lipids, enzymes, or peptides. Others created assemblies that grew but did not divide, or divided without inheriting structure. None had achieved the holy grail: spontaneous, inherited self-reproduction in a biochemistry-free system.
This system is fundamentally chemical. It is not alive. It has no genes, no metabolism, and no evolutionary machinery. Yet, it creates a cycle—a repeating pattern of self-assembly, growth, and reproduction. The vesicles, born of nothing more than designed molecules and light, became active agents in their own propagation. They were not static objects; they were chemical systems on the brink of life.
What’s more, the reproduction process was nonlinear and exponential. Each new vesicle generation released its own spores, leading to an explosive proliferation of synthetic “cells.” This mirrors biological reproduction, where population growth accelerates across generations.
A Glimpse into Life’s Deep Origins
Why is this so important? Because it nudges open a door to one of the deepest mysteries of science: How did life begin?
Modern life relies on incredibly complex machinery. But early Earth, four billion years ago, had none of it. How did lifeless molecules organize themselves into the first cells? How did self-replication begin before DNA and RNA?
This study offers a tantalizing clue. It suggests that reproduction itself may not require life—at least not in the way we traditionally define it. Instead, under the right conditions, simple chemistry may produce self-replicating structures that could eventually serve as scaffolds for more complex, evolving systems.
The polymeric vesicles created in this experiment don’t evolve—at least, not yet. But they show how key properties of life can arise spontaneously from nonliving matter. This bridges the gap between chemistry and biology, offering a new framework for thinking about the origin of life—not as a single moment of “creation,” but as a continuum from simplicity to complexity.
Beyond Origins: Toward Artificial Life
The implications extend far beyond early Earth. This work pushes the boundaries of synthetic biology into the domain of truly artificial life. It hints at the possibility of designing programmable, non-biological systems that can reproduce, adapt, and perhaps, one day, evolve.
Such systems could serve as foundations for smart materials, self-repairing devices, or even synthetic ecosystems. In space exploration, they could play a crucial role in terraforming or life-support systems, offering an alternative to fragile biochemistry in hostile environments.
There’s even a philosophical edge to this discovery. It challenges the notion that life requires carbon-based biochemistry. If reproduction and inheritance can arise in purely synthetic polymers, then life—as a phenomenon—may be far more universal than we imagined. Perhaps, somewhere in the cosmos, similar processes are playing out with alien chemistries.
Conclusion: The Spark Before Life
In the end, this study doesn’t create life—but it creates something hauntingly close. It demonstrates that self-reproduction, the engine of life, can emerge in the absence of life itself. This is not just a scientific curiosity; it is a profound insight into how nature might bootstrap itself from chaos to complexity.
Einstein once said, “The most beautiful thing we can experience is the mysterious.” The work of these researchers is a tribute to that sentiment. In a beaker of carefully chosen molecules, nudged by green light, they have conjured the ghost of life—proof that reproduction can begin even before life itself has taken form.
The cell, it seems, may not be the beginning of life. It might be just a chapter in a much larger story—a story that begins, as this one does, with a flicker of light and a spark of chemistry.
Reference: Sai Krishna Katla et al, Self-reproduction as an autonomous process of growth and reorganization in fully abiotic, artificial and synthetic cells, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2412514122