Humanity has always been haunted by questions of time. When we stumble upon an ancient spearhead buried in the soil, a fossilized bone glistening in stone, or a mysterious shard of pottery, our first instinct is wonder. Who touched this? When did they live? How long has this object slept beneath the earth? To answer these questions is to bridge the gap between past and present, to pull forgotten voices into the light of our modern age.
The science of dating ancient artifacts is more than measurement; it is the art of restoring context, of giving life back to silent objects. Every number—whether an age of 1,200 years or 3.2 million years—adds another line to the story of humanity and the planet. At the heart of this science lies radiocarbon dating, a method so revolutionary that it reshaped archaeology forever. Yet radiocarbon is only one chapter in a broader chronicle of techniques scientists use to decipher time. To truly understand the ages of ancient artifacts, we must look at carbon-14—and far beyond it.
The Birth of Radiocarbon Dating
The story begins in the 1940s, with a chemist named Willard Libby. During his work on the Manhattan Project, Libby became fascinated by the potential of radioactive isotopes to tell time. He proposed that living organisms, while alive, absorb carbon from the atmosphere, including a rare radioactive isotope known as carbon-14. When they die, they stop absorbing carbon, and the carbon-14 within their tissues begins to decay. By measuring the amount of carbon-14 left, one could estimate how long ago the organism died.
In 1949, Libby and his team announced their results: the ages of ancient wood, charcoal, and bone could be measured with remarkable accuracy. Suddenly, archaeologists had a clock they could carry into the field, a way to pin down prehistory with numbers rather than guesses. For this achievement, Libby received the Nobel Prize in Chemistry in 1960. His discovery gave rise to what we now call radiocarbon dating, or simply C-14 dating.
How Carbon-14 Works
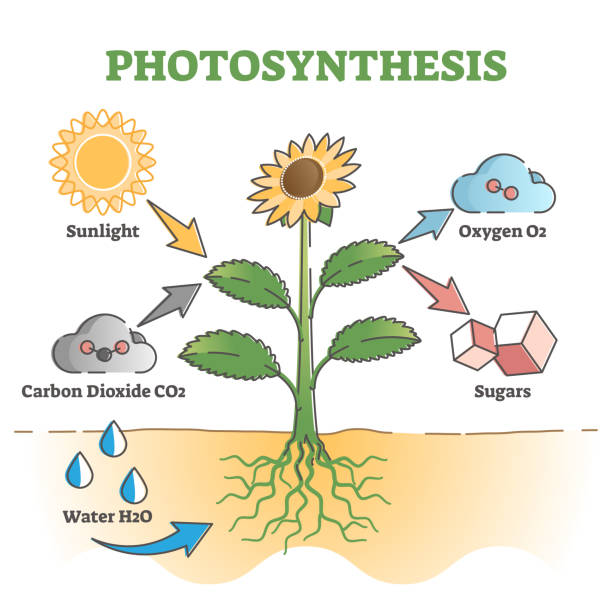
To grasp the magic of carbon-14, one must first understand isotopes. Carbon exists in three natural forms: carbon-12, carbon-13, and carbon-14. The first two are stable, but carbon-14 is unstable—it decays over time. It is formed high in the atmosphere when cosmic rays strike nitrogen atoms, converting them into carbon-14. This radioactive carbon then mixes with carbon dioxide, which is absorbed by plants through photosynthesis. Animals, in turn, eat the plants, and thus every living creature carries a trace of carbon-14 in its body.
The moment an organism dies, however, this exchange stops. The carbon-14 inside begins to decay into nitrogen-14 at a known rate, with a half-life of about 5,730 years. This means that after 5,730 years, half of the original carbon-14 is gone; after another 5,730 years, half of what remains is gone again, and so on. By comparing the ratio of carbon-14 to stable carbon isotopes in a sample, scientists can calculate the time since death.
The Power and Limits of Radiocarbon
Carbon-14 revolutionized archaeology, but it is not without limits. Because of its half-life, it is most effective for dating materials up to around 50,000 years old. Beyond that, the remaining carbon-14 is so faint that it becomes nearly indistinguishable from background noise. This makes C-14 perfect for human history and late prehistory but insufficient for the deeper time scales of evolution or geology.
Radiocarbon dating also requires organic material: bone, wood, charcoal, cloth, seeds, shells, or anything that once lived. Stone tools, metal weapons, or pottery shards cannot be directly dated with carbon-14 unless they carry traces of organic residue, such as food, soot, or bone. Moreover, the method is not immune to contamination. Modern carbon seeping into a sample can make it appear younger, while old carbon from groundwater or environmental factors can make it appear older.
Despite these challenges, refinements in technique—such as accelerator mass spectrometry (AMS)—have greatly improved precision. AMS allows scientists to count individual carbon-14 atoms, requiring much smaller samples and producing more accurate results. Today, a fragment of bone the size of a fingernail can yield a reliable radiocarbon age.
Calibration: The Quest for Accuracy
There is, however, another wrinkle in the story. The amount of carbon-14 in the atmosphere is not constant over time. Solar activity, volcanic eruptions, and human activity all influence the production of carbon-14. To correct for these fluctuations, scientists use calibration curves.
By comparing radiocarbon ages with independently dated records—such as tree rings (dendrochronology), corals, or lake sediments—researchers can adjust raw radiocarbon results to reflect true calendar ages. This process, called calibration, ensures that when we say a piece of wood is 3,200 years old, it truly reflects 3,200 years on the human calendar, not just on the radiocarbon clock.
Beyond Carbon-14: Other Radiometric Methods
Carbon-14 is only one tool in the scientist’s timekeeping arsenal. For artifacts and fossils older than 50,000 years, or for materials that never contained carbon at all, other radiometric techniques step in. These methods rely on the decay of different isotopes, each with its own half-life and applications.
Potassium-Argon and Argon-Argon Dating
When volcanic rocks solidify, they trap potassium-40, a radioactive isotope that decays into argon-40 with a half-life of 1.25 billion years. By measuring the ratio of potassium to argon, scientists can date volcanic layers surrounding artifacts or fossils. This method has been crucial in East Africa’s Great Rift Valley, where hominin fossils are often sandwiched between layers of volcanic ash. Argon-argon dating, a more refined version, offers even greater precision.
Uranium-Series Dating
Uranium isotopes, present in minerals like calcium carbonate, decay through a series of steps into stable lead isotopes. By measuring these ratios, scientists can date cave formations, corals, and even the calcite layers covering ancient paintings. Some of the world’s oldest cave art, tens of thousands of years old, has been dated this way, proving that art and symbolic thought were not unique to modern humans.
Thermoluminescence and Optically Stimulated Luminescence
When minerals like quartz or feldspar are exposed to sunlight or heat, electrons become trapped within their crystal structures. Over time, radiation from the environment builds up more trapped electrons. Heating or re-exposing the minerals to light releases these electrons, producing a measurable glow. Thermoluminescence and optically stimulated luminescence methods are used to date ceramics, burned flint, and sediments, providing ages up to several hundred thousand years.
Electron Spin Resonance
Similar in concept to luminescence, electron spin resonance measures trapped electrons in tooth enamel or shell. This method has dated human fossils too old for carbon-14, such as remains of early Homo sapiens in Africa and Neanderthals in Europe.
Fission Track Dating
When uranium atoms spontaneously split inside minerals, they leave microscopic scars called fission tracks. Counting these tracks, and comparing them with uranium content, provides ages for volcanic glass, zircon, and other minerals—useful for millions-of-years time scales.
Cosmogenic Nuclide Dating
Exposed rock surfaces are bombarded by cosmic rays, which generate rare isotopes like beryllium-10 or aluminum-26. Measuring these isotopes reveals how long a rock has been exposed to the atmosphere, useful for reconstructing glacial advances and retreats.
Dendrochronology: The Tree-Ring Clock
While radiometric methods dominate, some of the most precise dating comes from the humble tree. Trees grow annual rings that record not only age but also environmental conditions. By matching overlapping ring patterns from living and ancient trees, scientists have built continuous records stretching back over 12,000 years.
Dendrochronology is a powerful tool on its own, but it also serves as the backbone for radiocarbon calibration. Each ring provides a snapshot of carbon-14 levels at a specific time, creating an invaluable reference for correcting radiocarbon dates.
The Interdisciplinary Web of Dating
Dating ancient artifacts is never a matter of one method alone. Archaeologists often weave together multiple lines of evidence, cross-checking results for accuracy. A piece of pottery might be dated by thermoluminescence, while charcoal from the same layer is dated by radiocarbon. Volcanic ash above or below provides potassium-argon ages, anchoring the timeline in geological context.
The interplay of methods transforms archaeology from guesswork into science. It allows us to build chronologies that are both precise and meaningful, linking cultural events with environmental changes, migrations, and evolutionary milestones.
Famous Cases: Dating History
Several landmark discoveries showcase the power of dating techniques. The Dead Sea Scrolls, fragile parchments discovered in caves, were dated by radiocarbon, confirming their origins in the centuries around the birth of Christianity.
The famous “Iceman,” Ötzi, found frozen in the Alps, was dated to about 5,300 years ago through radiocarbon analysis of his clothing and body tissues. This placed him in the Copper Age, transforming our understanding of prehistoric Europe.
In paleoanthropology, potassium-argon dating has provided ages for hominin fossils like “Lucy,” the Australopithecus afarensis skeleton from Ethiopia, dated to 3.2 million years old. Without these methods, Lucy might still be just a fossil without a place in time.
The Human Story in Numbers
Every artifact, once dated, becomes a point on the vast map of human history. The numbers reveal not only when but also how civilizations rose and fell, how humans migrated across continents, how climate shaped survival.
Dating is what allows us to say that Stonehenge was built around 4,500 years ago, that the first cities in Mesopotamia flourished around 6,000 years ago, that Homo sapiens left Africa around 60,000 years ago. It turns myths into timelines, legends into evidence, stories into science.
The Future of Dating Technologies
The science of dating is still evolving. New methods push accuracy further, while advances in instruments allow analysis of ever smaller samples. Accelerator mass spectrometry continues to refine radiocarbon dating, while synchrotron radiation and high-resolution imaging reveal atomic-scale details in minerals.
Emerging fields like proteomics (the study of ancient proteins) and isotopic fingerprinting promise new ways to place artifacts in time. Meanwhile, improved calibration records—from ice cores, corals, and sediments—extend the reach of existing techniques.
One tantalizing frontier is astrobiology. If we ever discover life beyond Earth, whether on Mars or icy moons, dating its remnants will be essential to understanding its history. The tools we refine today on Earth may one day measure the ages of alien fossils.
Time, Memory, and Meaning
Ultimately, the science of dating ancient artifacts is not just about numbers—it is about meaning. Every measurement places us in relation to our ancestors and to the planet itself. It allows us to see humanity not as a timeless abstraction but as a species that has struggled, adapted, and thrived across millennia.
Carbon-14 was the beginning, the spark that gave voice to silent bones and ashes. Beyond it, an array of methods extends our gaze deeper into time, illuminating epochs long before written words or memory. Together, they form a scientific symphony that restores context to the fragments of the past.
To hold an ancient artifact is to hold mystery in your hands. To date it is to whisper to it, Tell me your story. Through the patient work of science, the artifact replies—not in poetry, but in numbers. Yet those numbers, once placed upon the canvas of history, become a kind of poetry after all: the poetry of time, memory, and the enduring human desire to know where we come from.