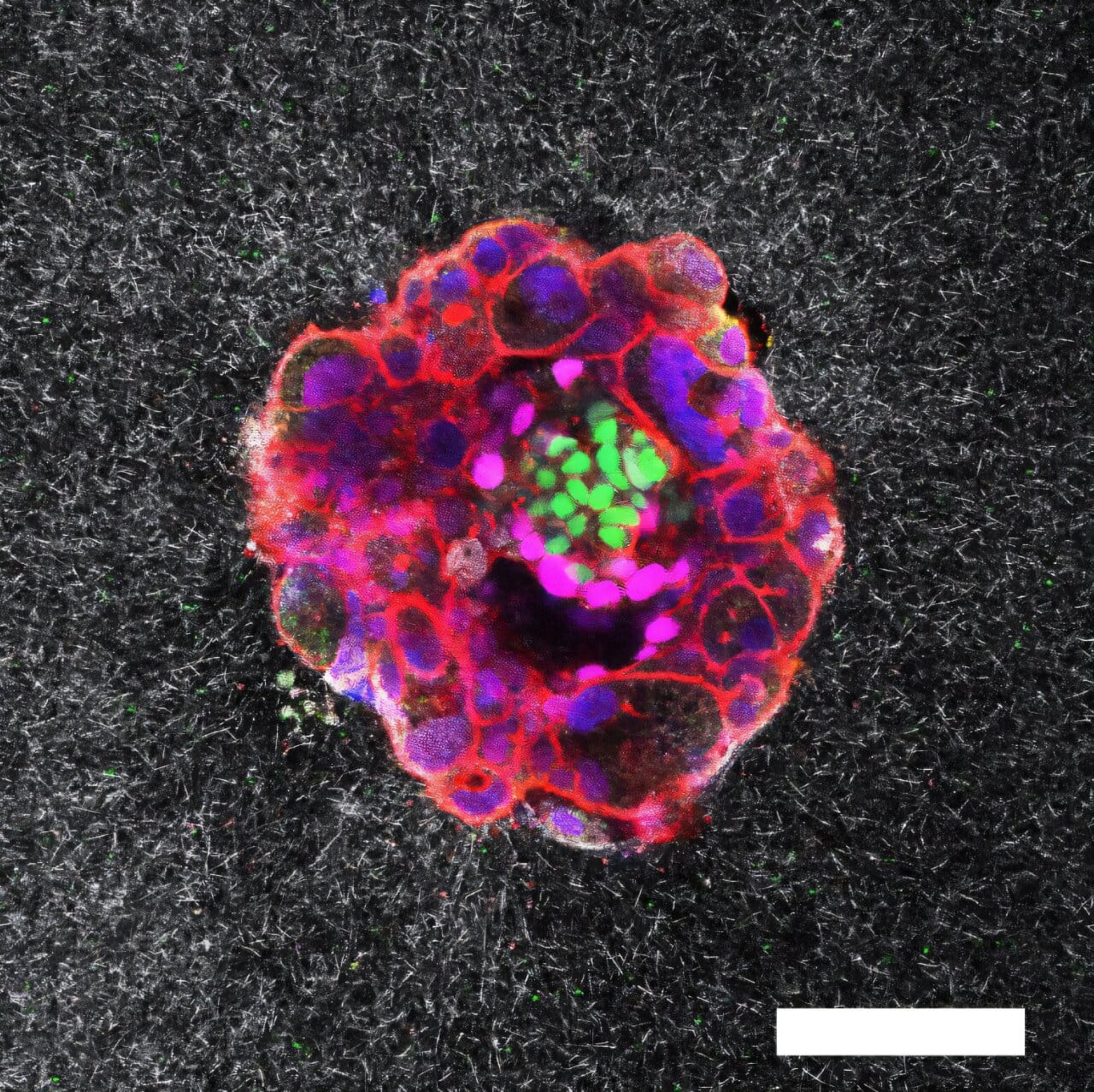
For the first time in history, scientists have peered into one of the most intimate and mysterious moments of human life: the instant a tiny embryo burrows into the uterus, beginning the journey toward pregnancy. Researchers at the Institute for Bioengineering of Catalonia (IBEC), in collaboration with Dexeus University Hospital, have captured this elusive process in unprecedented detail, recording it in real time and in three dimensions.
Their groundbreaking work, published in Science Advances, not only satisfies a century-long scientific curiosity but also opens the door to new insights into infertility, one of the most pressing health challenges of our time.
The Challenge of Implantation
Conception is often portrayed as the miraculous fusion of sperm and egg, but in reality, the story is far from over at fertilization. For pregnancy to begin, the embryo must implant successfully into the lining of the uterus. And this step is anything but simple.
Implantation failures are the leading cause of infertility and account for up to 60% of spontaneous miscarriages. Yet, despite its central role, implantation has remained almost invisible to science. Until now, researchers could only rely on still images captured at isolated moments, leaving the process itself a mystery.
“This is the first time anyone has observed, in real time, how a human embryo invades the uterus,” explains Samuel Ojosnegros, principal investigator of IBEC’s Bioengineering for Reproductive Health group and lead author of the study. “What we found is that it’s not a passive event. The embryo actively exerts force, burrowing its way inside. It’s a surprisingly invasive and dynamic process.”
A Forceful Entry
The images revealed a process both delicate and powerful. As the embryo approaches the uterine lining, it releases enzymes that break down surrounding tissue, softening a path forward. But chemistry alone is not enough. The embryo also uses mechanical force to push and pull its way deeper into the uterus.
The uterine lining is no gentle surface. It is rich in collagen, a fibrous protein that gives structure to tendons, cartilage, and connective tissue. To survive, the embryo must remodel this dense network, tugging and reorganizing it as it presses inward.
“We observed that embryos pull on the uterine matrix, reorganizing it as they move,” says Amélie Godeau, co-first author of the study. “They’re not only reacting to the tissue environment; they’re shaping it. It’s a dialogue between embryo and uterus, guided by mechanical forces.”
This remodeling does more than clear a path. It lays the foundation for one of the most critical partnerships in biology: the connection between the embryo and the mother’s blood supply. As the embryo secures itself, it begins to form specialized tissues that will eventually become the placenta, a life-sustaining organ that nourishes the growing fetus.
A Laboratory Window into Life’s Origins
To achieve this breakthrough, the IBEC team designed a novel laboratory platform that recreates the uterine environment outside the body. Using a gel made of collagen and key proteins, they built an artificial matrix in which embryos could implant under controlled conditions.
This setup allowed researchers to track implantation using real-time fluorescence imaging, capturing both the chemical and mechanical interactions between embryo and tissue. To better understand the uniqueness of human implantation, they also studied mouse embryos, revealing stark differences between species.
“In mice, the uterus folds around the embryo, forming a protective crypt,” explains Anna Seriola, IBEC researcher and co-first author. “But in humans, the embryo takes a more aggressive approach, invading directly into the uterine tissues and expanding outward from within. These differences highlight just how specialized human reproduction really is.”
Why This Discovery Matters
Beyond its scientific beauty, this discovery carries profound implications for reproductive medicine. By quantifying the “mechanical footprint” of implantation, the study offers new clues into why pregnancies sometimes fail at this earliest stage.
“Understanding how embryos exert and respond to force could help us refine fertility treatments,” says Ojosnegros. “If we can identify the conditions that support successful implantation, we may be able to improve embryo selection, increase the chances of conception, and reduce the emotional and physical burden of infertility.”
For the millions of people worldwide who struggle to conceive, this is more than academic progress. It is hope.
A New Chapter in Reproductive Science
The IBEC team’s findings remind us of a simple truth: even the smallest beginnings are shaped by struggle and resilience. The embryo, barely a cluster of cells, does not wait passively for life to welcome it. It digs, pulls, and fights its way into the world, transforming both itself and its environment in the process.
For centuries, implantation has been a hidden chapter of human development, a silent moment that even the most advanced microscopes could not fully capture. Today, thanks to innovation, patience, and a willingness to look closely, science has turned the invisible visible.
And in doing so, it has revealed not just how life begins, but how fiercely it insists on beginning.
More information: Traction force and mechanosensitivity mediate species-specific implantation patterns in human and mouse embryos, Science Advances (2025). DOI: 10.1126/sciadv.adr5199