Every time you scrape your knee, nick your finger, or suffer a cut, something remarkable happens beneath the surface. Cells near the wound don’t simply sit idle—they immediately mobilize, sensing the damage and working together to close the gap. To the naked eye, this looks like a scab forming and new skin growing. But on the microscopic scale, it is a breathtaking choreography of living matter, cells pulling, crawling, and reshaping themselves with astonishing precision.
For years, scientists have known that the geometry of a wound—the curve of its edges—affects how skin cells migrate to heal it. Yet the deeper mystery remained: How could something as subtle as the shape of a wound edge, barely visible under a microscope, dictate the very way a cell decides to move?
Convex or Concave: Two Different Paths to Healing
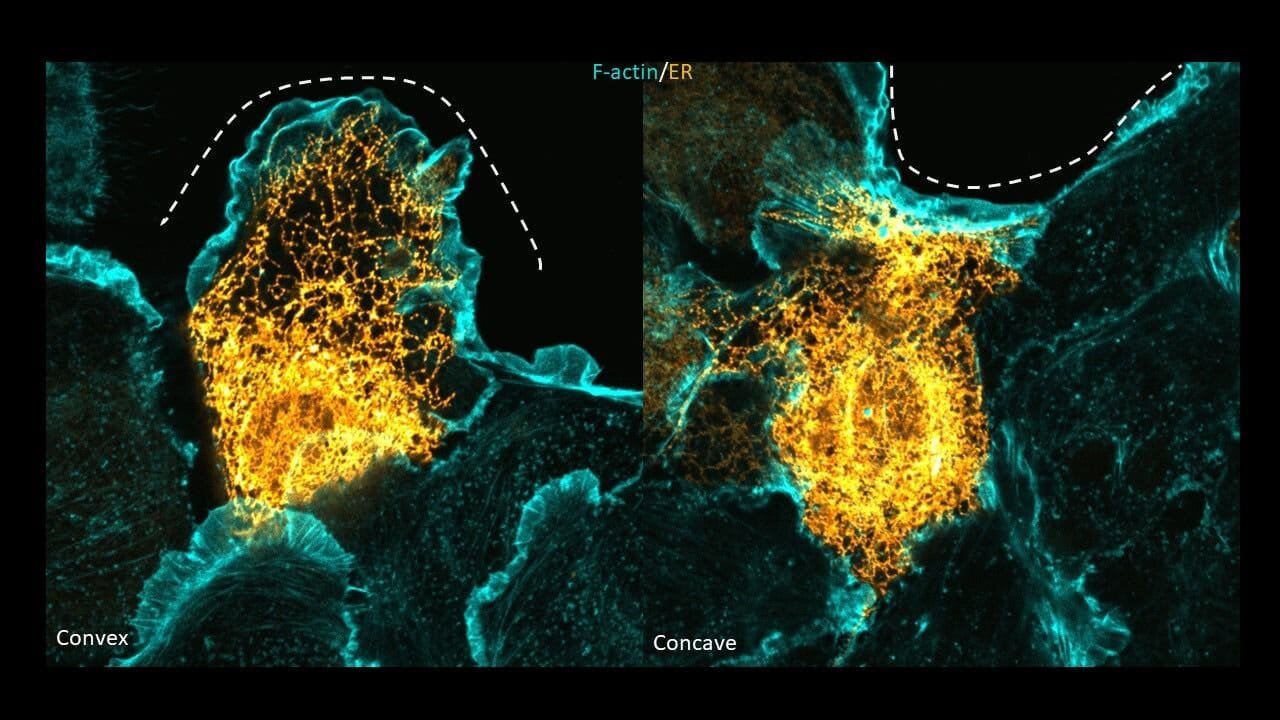
Imagine a wound shaped like a shallow bowl (concave) or a small bump (convex). At a convex edge, skin cells tend to grow finger-like protrusions and crawl individually into the gap. But at concave edges, they behave differently: instead of spreading out, they tighten the edge of the wound collectively, like a drawstring pulling a purse closed.
These contrasting behaviors—crawling versus contracting—had long been documented. But the reason behind this split personality was elusive. Why should a tiny curve in the edge of a wound cause cells to change strategy so drastically?
A Student’s Curiosity Unlocks a Mystery
Four years ago, Simran Rawal, then a graduate student in the laboratory of Tamal Das at the Tata Institute of Fundamental Research in Hyderabad, decided to follow this puzzle. Rather than just watching cells move, she wanted to know what was happening inside them as they responded to wounds of different shapes.
Her persistence led to a discovery recently published in Nature Cell Biology. The answer lay in one of the most overlooked, yet essential, organelles of the cell: the endoplasmic reticulum (ER).
The Endoplasmic Reticulum Steps Into the Spotlight
The ER is the largest organelle inside our cells, a sprawling network of membranes usually known for protein synthesis and calcium signaling. Most biology textbooks describe it as the cell’s factory floor, quietly churning out proteins and lipids. Yet Rawal’s work revealed an unexpected side of the ER: it acts as a shape-sensing organelle.
When cells at the edge of a wound face a convex curve, their ER restructures into long, thin tubes. At concave curves, however, the ER flattens into broad sheets. This seemingly subtle transformation has profound consequences: it determines whether the cell crawls or contracts to help close the wound.

The ER, in other words, is not a passive factory. It is an active decision-maker in how cells respond to physical geometry.
Switching Strategies by Reshaping the ER
To test the ER’s role, Rawal and colleagues performed a bold experiment. They forced cells at concave edges—where the ER normally flattens into sheets—to restructure their ER into tubular shapes. The result was stunning. Instead of contracting the wound edges as usual, these cells switched behavior, sprouting protrusions and crawling forward.
This proved that ER morphology is not just correlated with wound-healing behavior—it is directly responsible for determining it.
The Cytoskeleton and ER: Partners in Motion
The ER doesn’t act alone. Its transformations are guided by the cytoskeleton, the dynamic scaffolding made of actin filaments and microtubules that gives cells their shape and mobility.
At convex wound edges, microtubules play a key role in shaping the ER into tubes. At concave edges, both actin and microtubules coordinate to flatten the ER into sheets. These partnerships allow the ER to sense mechanical cues from the environment and reorganize itself accordingly.
Measuring the Mechanics of Healing
But why go through the trouble of switching between crawling and contracting? To answer this, the team collaborated with physicist Pradeep Keshavanarayana from the University of Birmingham, UK. Using mathematical models, he calculated the mechanical “strain energy” cells experience when they move in different ways.
The results revealed a simple truth: cells want to minimize strain. On convex surfaces, crawling with protrusions lowers the energy cost. On concave surfaces, contracting together as a team reduces strain more efficiently. The ER’s shape changes act as the crucial switch, allowing cells to pick the strategy that saves the most energy while still healing the wound.
The ER as a Hidden Mechanotransducer
This discovery reframes how we think about the ER. For decades, the cytoskeleton was considered the primary sensor of mechanical forces in cells. But Rawal’s work shows that organelles themselves, especially the ER, can act as mechanotransducers—translating physical cues into biochemical signals that ripple throughout the cell.
Because the ER stretches from the nuclear envelope to the cell periphery, any structural change in its architecture can influence processes across the entire cell. It is not hard to imagine how such a mechanism could play roles beyond wound healing—perhaps in tissue development, organ repair, or even cancer progression.
A New Dimension in Wound Healing
Most past studies of wound healing focused on biochemical signals—growth factors, signaling molecules, protein cascades. But this research highlights a surprising new player: geometry itself. The shape of a wound isn’t just a passive outcome of injury—it actively instructs cells on how to move.
By uncovering the ER’s role in sensing curvature and guiding behavior, this study adds a new layer of complexity to the story of how our bodies repair themselves. It also sparks big questions. Could other organelles act as shape sensors too? Could manipulating ER morphology accelerate wound healing in medical treatments? Could similar principles guide how tissues form in an embryo or regenerate after injury?
Healing as a Symphony of Forces
Wound healing, then, is not simply a chemical story—it is a symphony of mechanics, geometry, and cellular architecture. The cells at the edge of a wound are not blindly crawling forward; they are engineers, measuring shapes, reorganizing their machinery, and choosing the most efficient way to close the gap.
Simran Rawal’s discovery gives us a glimpse into this hidden intelligence of cells. It shows that even the smallest details—a curve in a wound, a shift in organelle shape—can ripple outward to determine the success of healing.
Beyond the Lab: Why It Matters
For the millions of people suffering from chronic wounds, burns, or tissue injuries, understanding these processes is more than academic curiosity. If scientists can learn to guide or mimic the ER’s behavior, it may open doors to new therapies that speed up healing or improve tissue repair.
More broadly, this study is a reminder of the profound intelligence woven into our bodies. Every cut and scrape sets in motion a microscopic ballet that most of us never notice. Yet behind this everyday miracle lies a complex dialogue between geometry, mechanics, and biology.
The Shapes That Heal Us
Einstein once said that nature hides her secrets in simplicity. Here, the simple curve of a wound edge reveals a hidden world where organelles listen to geometry and decide how to heal us. The ER, once thought of as a mere protein factory, emerges as a conductor in this orchestra of repair.
The next time you watch a small wound on your skin vanish over days, remember: it wasn’t just chemistry at work. It was also geometry—the bend of a curve whispering instructions to cells, and the quiet reshaping of organelles deciding how best to stitch you back together.
More information: Rawal, S. et al. Edge curvature drives endoplasmic reticulum reorganization and dictates epithelial migration mode, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01729-3, www.nature.com/articles/s41556-025-01729-3