In the race toward a sustainable future, few natural materials hold as much promise—and present as much challenge—as cellulose. This robust, glucose-based polymer is the most abundant renewable organic compound on Earth, forming the sturdy framework of plant cell walls. And yet, despite its potential as a feedstock for biofuels and green chemicals, cellulose has long stood as a stubborn gatekeeper—resistant to degradation, even in nature.
Its crystalline microfibrillar architecture, tightly bound to lignin and hemicellulose, renders it extremely recalcitrant to enzymatic attack. Traditional enzymes, even those tailored by evolution over millennia, struggle to break it down efficiently. This bottleneck has been one of the great barriers to turning agricultural waste into high-yield biofuels, such as second-generation ethanol. But a remarkable scientific breakthrough has now shattered that barrier—and with it, a decades-old paradigm.
A Game-Changing Discovery from Brazil
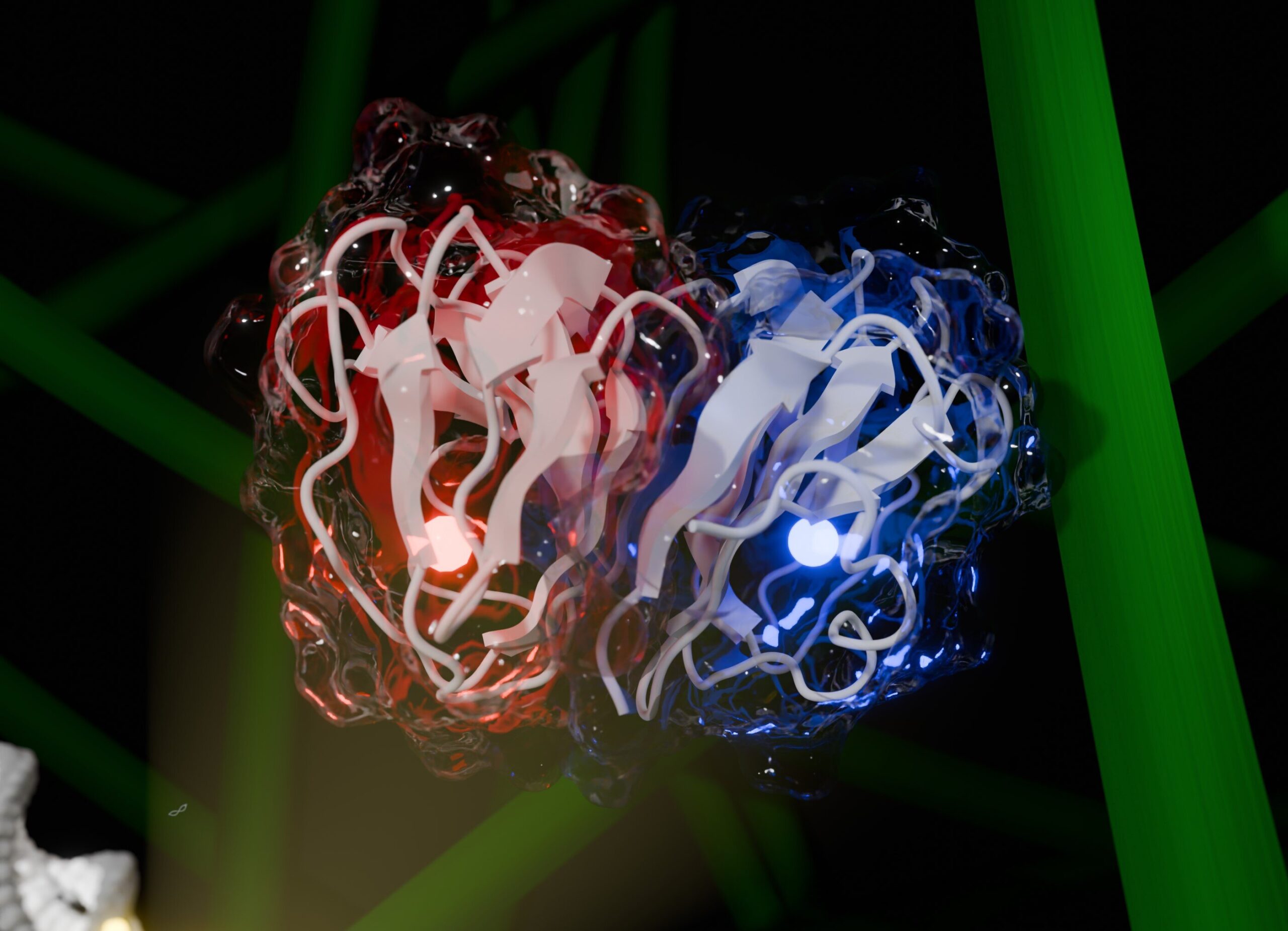
In a landmark study published in Nature, scientists from the Brazilian Center for Research in Energy and Materials (CNPEM), alongside international collaborators, unveiled a newly discovered enzyme that could revolutionize biomass conversion. Named CelOCE—short for cellulose oxidative cleaving enzyme—this metalloenzyme has introduced an entirely new mechanism for breaking down cellulose, setting the stage for more efficient biofuel production on a commercial scale.
“We’ve identified a metalloenzyme that enhances cellulose conversion through a previously unknown mechanism of substrate binding and oxidative cleavage,” explains Mário Murakami, head of CNPEM’s Biocatalysis and Synthetic Biology Group and lead author of the study. “This discovery establishes a new frontier in redox biochemistry for the depolymerization of plant biomass, with broad implications for biotechnology.”
CelOCE: The Master Key to Cellulose
To understand CelOCE’s transformative role, imagine cellulose as a series of locked chains—intertwined, fortified, and inaccessible to traditional enzymatic tools. Most enzymes act like cutters, capable of snipping the chains if they can find an entry point. But CelOCE acts like a locksmith, breaking open the locks themselves.
Its role isn’t to finish the job but to make the cellulose accessible, effectively preparing the material for a full enzymatic assault. “There’s a synergy,” says Murakami. “CelOCE potentiates the action of other enzymes by disrupting the structure at critical junctures.”
This unique capability arises from a previously unseen oxidative cleavage mechanism—a feature that sets CelOCE apart from the monooxygenases that once reigned supreme in cellulose degradation science.
Breaking the Redox Paradigm
Two decades ago, the first major leap in biomass deconstruction came with the discovery of monooxygenases—enzymes that deploy redox chemistry to cleave glycosidic bonds in cellulose, accelerating degradation. That shift was seen as a turning point, and for years, monooxygenases were considered nature’s ultimate answer to cellulose recalcitrance.
But CelOCE has shattered that assumption. It is not a monooxygenase—and yet, it doubles the effect of current enzyme cocktails. “We’ve changed the paradigm,” Murakami says. “We thought monooxygenases were the only redox solution, but nature had a better strategy up its sleeve—leaner, more efficient, and more adaptable.”
CelOCE targets the ends of cellulose fibers, latches on, and begins its work. As a dimer (a protein made of two identical subunits), it performs two coordinated tasks: one subunit binds and cleaves the cellulose, while the other produces hydrogen peroxide—a crucial co-substrate for the reaction.
Self-Powered Chemistry: A Catalytic Marvel
One of CelOCE’s most astonishing features is its self-sufficiency. Unlike monooxygenases, which require an external supply of hydrogen peroxide—a reactive and difficult-to-control compound—CelOCE generates its own peroxide in situ.
“This is a huge advantage,” Murakami notes. “Hydrogen peroxide is tricky. It’s reactive, potentially harmful, and hard to manage in large-scale processes. But CelOCE produces exactly what it needs, when and where it needs it. It’s a complete catalytic machine.”
This self-contained system is made possible by the enzyme’s quaternary structure, which enables one subunit to carry out cleavage while the other supports the reaction by producing the oxidant. The copper atom at CelOCE’s core—its catalytic center—classifies it as a metalloenzyme, granting it powerful redox capabilities.
From Soil Sample to Industrial Pilot: The Journey of Discovery
CelOCE wasn’t synthesized in a lab—it was discovered in nature, hidden in plain sight. Researchers began their hunt in soil samples from sugarcane bagasse fields—land rich with microbial life accustomed to breaking down plant material. Using a battery of cutting-edge tools, the team uncovered the microbial community responsible for this natural decomposition.
Their investigation combined:
- Metagenomics and proteomics to map microbial DNA and proteins
- Carbohydrate enzymology to characterize activity
- Fourth-generation synchrotron X-ray diffraction for atomic-scale structure analysis
- Fluorescence and absorption spectroscopy for chemical profiling
- Site-directed mutagenesis to tweak and test enzyme function
- CRISPR/Cas gene editing to engineer production strains
- Pilot bioreactors (65L to 300L) to demonstrate industrial viability
In other words, they didn’t just find a molecule—they mapped its ecosystem, deciphered its mechanism, and demonstrated how it could scale from petri dish to industrial pipeline.
A Catalyst for Change: Real-World Impact
One of the most remarkable aspects of this discovery is its readiness for deployment. CelOCE has already been tested in pilot-scale facilities, and its integration into current biofuel production systems is immediately feasible.
This is especially critical for Brazil, home to the only two commercial-scale biorefineries producing ethanol from cellulose. With hundreds of millions of tons of sugarcane bagasse, corn stover, and other agro-waste available each year, even small improvements in conversion efficiency translate to massive gains in output.
Murakami estimates current cellulose conversion rates hover around 60–70%, occasionally reaching 80%. But CelOCE could boost yields significantly, unlocking resources that currently go unused. The implications extend beyond ethanol, touching everything from aviation biofuels to bioplastics and green chemicals.
A Glimpse Into the Future of Biomass Utilization
Beyond its immediate industrial utility, CelOCE also offers exciting avenues for broader applications. Its minimalist structure, high efficiency, and redox flexibility make it a candidate for redesign in other fields, including:
- Environmental bioremediation: Breaking down persistent organic pollutants
- Waste valorization: Converting urban or marine biomass into usable products
- Bioplastics production: Generating fermentable sugars for biopolymer synthesis
With CelOCE, scientists have not only found a better way to crack cellulose—they’ve uncovered a new language in nature’s molecular toolkit, one that may hold solutions to many of our most pressing environmental and energy challenges.
Conclusion: The Enzyme That Could Power a Green Revolution
The discovery of CelOCE is more than a scientific achievement—it’s a strategic victory in the fight against climate change, a testament to the power of biodiversity, and a case study in the synergy of biology, chemistry, and engineering.
As biorefineries begin to incorporate this enzyme into their processes, we may witness a new era in renewable fuel production, where waste becomes wealth and cellulose—once the most intractable obstacle—becomes a cornerstone of clean energy.
In a world urgently searching for sustainable solutions, CelOCE is proof that sometimes, the key to unlocking the future lies in a tiny molecule with an extraordinary talent—waiting patiently in the soil.
Reference: Clelton A. Santos et al, A metagenomic ‘dark matter’ enzyme catalyses oxidative cellulose conversion, Nature (2025). DOI: 10.1038/s41586-024-08553-z