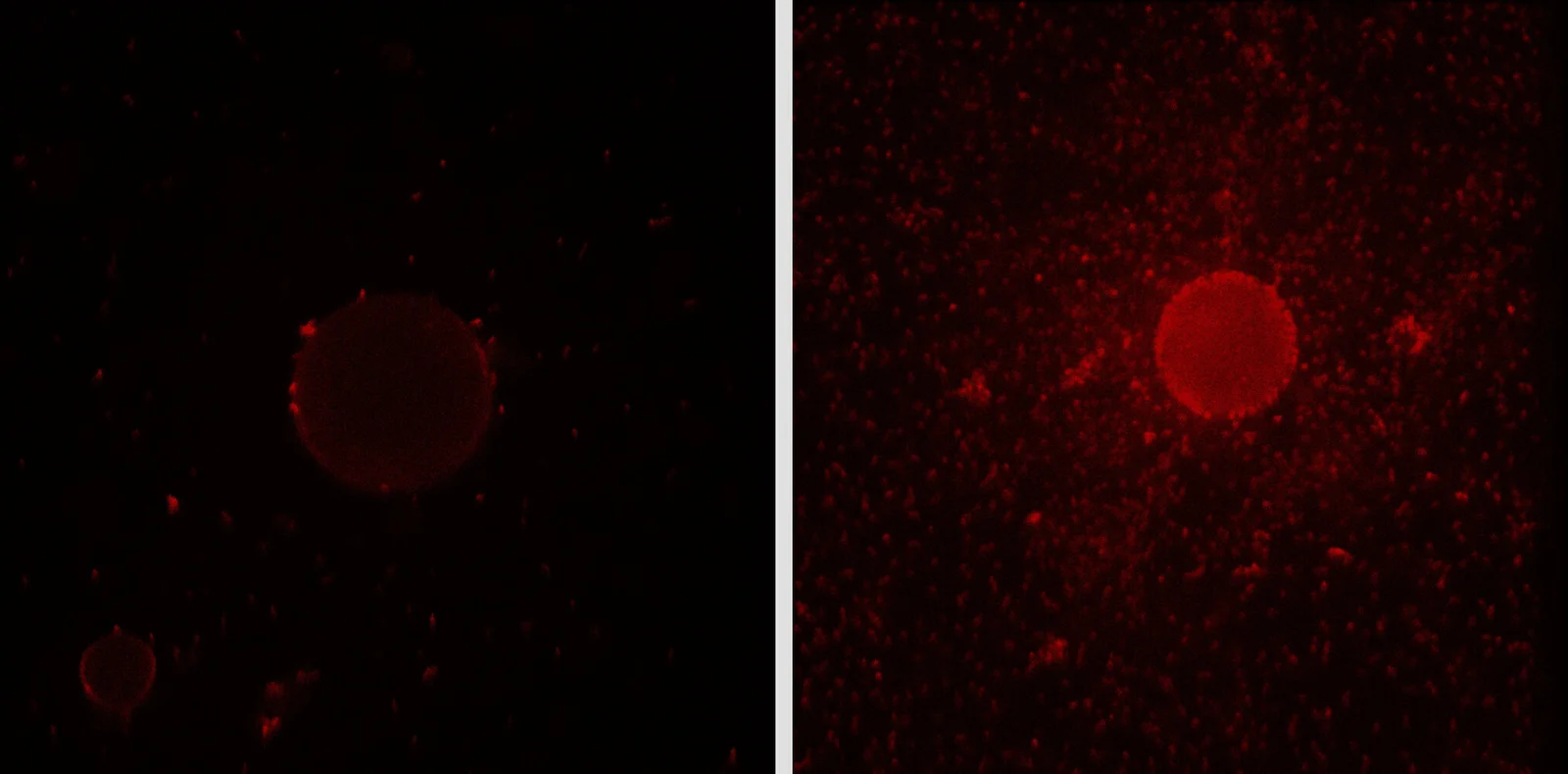
Beneath the surface of our oceans, beyond the sight of satellites and sonar, lives a microscopic hero with a voracious appetite for pollution. Its name is Alcanivorax borkumensis, and it is no ordinary microbe. This marine bacterium thrives in the aftermath of environmental disasters, emerging like a microbial fire brigade at the scene of oil spills. Unlike most bacteria that struggle in such toxic environments, A. borkumensis grows rapidly, attaching itself to oil droplets and beginning the breakdown process within hours.
But how does this tiny organism accomplish such a feat in a world where oil and water famously refuse to mix? The answer lies in biochemistry as elegant as it is effective: the bacterium produces its own version of dish soap—a biosurfactant—that lets it cling to oily surfaces, making hydrocarbon consumption possible. Now, thanks to groundbreaking research published in Nature Chemical Biology, scientists have unraveled the exact mechanism behind this natural marvel—a discovery that could revolutionize bioremediation and green biotechnology.
A Gourmet for Hydrocarbons
To understand why Alcanivorax borkumensis is uniquely equipped to combat oil spills, we must first look at its diet. This bacterium feeds on alkanes—long chains of hydrocarbons that are abundant in crude oil. These chains are packed with energy, making them a desirable food source if one can manage the greasy logistics.
The name Alcanivorax borkumensis translates roughly to “alkane devourer from Borkum,” referencing Borkum Island in the North Sea, near where it was first isolated. In its natural state, A. borkumensis is a rare player in the microbial soup of the sea. However, after an oil spill, it suddenly multiplies, sometimes forming over 90% of the bacterial population in the affected area.
This explosion in numbers is not just opportunistic—it’s functional. By coating oil droplets in biofilms and digesting hydrocarbons, A. borkumensis dramatically accelerates the breakdown of pollutants. But to do that, it must first overcome a fundamental challenge: oil and water don’t mix.
Dish Soap, Microbial-Style
How does A. borkumensis bridge the molecular divide between oil and water? It makes its own emulsifier—a naturally occurring biosurfactant. This compound is a hybrid molecule, part water-soluble, part fat-soluble, similar to the surfactants in everyday dishwashing liquid. It acts like a molecular diplomat, bringing together the incompatible worlds of hydrophilic and hydrophobic chemistry.
The biosurfactant consists of glycine (a simple amino acid) and a sugar-fatty acid compound. Together, they allow the bacterium to anchor itself on oil droplets, form a stable biofilm, and digest the hydrocarbons underneath. “The molecules have a water-soluble part and a fat-soluble part,” explains Professor Peter Dörmann, a biochemist at the University of Bonn. “The bacteria settle on the surface of the oil droplets, where they form a biofilm.”
This biofilm isn’t just a passive coating—it is a dynamic zone of molecular activity where hydrocarbons are broken down and absorbed. Without this biochemical innovation, the bacteria would remain adrift in the water, unable to access their carbon-rich feast.
Cracking the Genetic Code
Despite the bacterium’s ecological importance, the genetic basis of its biosurfactant production remained a mystery for years. That changed when a consortium of researchers from the University of Bonn, RWTH Aachen University, Heinrich Heine University Düsseldorf, and Forschungszentrum Jülich took a deep dive into its genome.
Led by Professor Karl-Erich Jaeger, the team identified a specific gene cluster responsible for the synthesis of the biosurfactant. By experimentally disabling these genes, they discovered that the bacteria lost their ability to attach to oil droplets and experienced a dramatic decline in growth. “As a result, they absorbed less oil and grew much more slowly,” said Professor Lars Blank of RWTH Aachen.
The implications were immediate and profound: this gene cluster, previously buried in the bacterium’s genetic library, held the key to understanding how A. borkumensis functions during oil spills.
Engineering Microbial Cleaners
Taking the research a step further, Jiaxin Cui, a doctoral student in Dörmann’s lab, decoded the biosynthetic pathway involved. It turns out the detergent molecule is assembled in a three-step enzymatic process involving a trio of specialized enzymes. Each enzyme catalyzes a specific chemical reaction, adding another layer to the detergent molecule.
Once the genes responsible for these enzymes were isolated, the researchers transferred them into a different species of bacterium. Astonishingly, this new host organism also began producing the detergent, proving that the process could be replicated outside its native species.
This synthetic biology breakthrough hints at the potential for genetically engineered microbes tailored for rapid oil spill cleanup or industrial use. By breeding strains with optimized versions of the biosurfactant-producing genes, scientists could significantly enhance oil degradation in marine ecosystems.
Bioremediation and Beyond
The implications of this research extend far beyond marine ecology. In a world increasingly reliant on petrochemicals—and increasingly plagued by their environmental consequences—finding sustainable ways to manage hydrocarbon pollution is urgent. A. borkumensis and its engineered relatives could become living tools in our battle against oil spills, tar sands runoff, and industrial waste.
But the applications don’t stop at cleanup. “This natural detergent could have biotech applications as well, such as for microbial production of key chemical compounds from hydrocarbons,” notes Professor Dörmann. In essence, the molecule that enables oil digestion might also be used to produce valuable chemicals for industrial and pharmaceutical purposes.
Imagine bacteria engineered not just to clean up pollution, but to convert it into useful products—bioplastics, biofuels, or medical precursors. That’s the transformative promise of this discovery.
Sustainable Futures in a Drop of Oil
The research is part of the University of Bonn’s Transdisciplinary Research Area (TRA) “Sustainable Futures,” a collaborative initiative focused on solving global challenges through innovation and interdisciplinary science. In that context, the story of Alcanivorax borkumensis serves as both a symbol and a solution—a reminder that nature, when properly understood, often holds the key to its own healing.
With global oil consumption still exceeding 90 million barrels per day and oil spills remaining a grim reality, the need for effective, low-impact remediation strategies is urgent. Chemical dispersants, long used in oil cleanup, come with their own ecological downsides. Microbial bioremediation offers a gentler, more sustainable alternative.
Thanks to the molecular detective work of this multinational research team, we are now closer than ever to unlocking the full potential of microbial oil eaters. What was once nature’s quiet cleanup crew is now poised to become a bioengineered force for environmental restoration.
A Microscopic Marvel with Macroscopic Impact
It’s easy to overlook a single-celled organism, especially one floating in the vastness of the ocean. Yet Alcanivorax borkumensis is a reminder that the smallest life forms can have the biggest impact. Armed with a natural detergent and an extraordinary genetic toolkit, this marine bacterium doesn’t just survive in polluted environments—it thrives in them, transforming toxic oil into harmless byproducts.
With modern science now illuminating its secrets, A. borkumensis may soon play an even bigger role in our future—helping to restore damaged ecosystems, innovate in biotechnology, and maybe even teach us a thing or two about resilience in the face of adversity.
In every oil droplet it digests, it performs a microscopic miracle. And thanks to the power of discovery, that miracle is one step closer to becoming a cornerstone of environmental science.
Reference: Jiaxin Cui et al, Biosurfactant biosynthesis by Alcanivorax borkumensis and its role in oil biodegradation, Nature Chemical Biology (2025). DOI: 10.1038/s41589-025-01908-1