For decades, modern medicine has advanced through the power of injections. From life-saving insulin to the revolutionary COVID-19 vaccines, needles have been the standard route for delivering complex drugs into the body. But with every jab comes a familiar tension—a clenched jaw, averted eyes, a deep breath before the sting. Injections are not just inconvenient; for many, they’re a genuine source of anxiety.
Beyond the emotional discomfort, injections pose practical challenges. They typically require trained professionals, sterile environments, and careful disposal of sharps—obstacles that slow the rollout of treatments during pandemics and make chronic self-dosing cumbersome. Imagine a person with an inflammatory bowel disease who needs repeated mRNA therapies. Each dose currently means another appointment, another injection, another disruption to daily life.
Researchers have long dreamed of a different future: one where swallowing a pill could deliver the same sophisticated therapeutics that usually demand a needle. For patients targeting diseases in the gut, especially, oral delivery seems natural. But achieving it has been far from simple.
The Harsh Journey Through the Digestive Tract
The challenge lies in the biology of digestion itself. When you swallow a pill, it plunges into a chemical storm. Stomach acid breaks down proteins and nucleic acids with ruthless efficiency. Enzymes shred foreign molecules to protect the body from pathogens. Intestinal mucus acts as a sticky shield, preventing large, fragile molecules like mRNA from slipping through to reach their targets.
Scientists have tried to shield mRNA using nanoparticles—tiny molecular carriers designed to protect and transport cargo through the gastrointestinal (GI) tract. While this approach offered some protection, most formulations disintegrated before reaching the small intestine, where absorption could occur. This limitation meant that oral mRNA therapies, once thought promising, never moved beyond concept.
Now, researchers at Harvard Medical School and Brigham and Women’s Hospital have changed the game.
A Smart Capsule with a Genetic Mission
In a groundbreaking study published in Science Translational Medicine, scientists unveiled an engineered ingestible capsule called RNACap, designed to safely ferry liquid mRNA through the harsh digestive environment and deliver it directly to intestinal cells.
Unlike standard mRNA therapeutics that rely on frozen storage and needle injections, RNACap offers a simpler, more patient-friendly approach. The capsule uses a pH-sensitive outer coating that shields it from the stomach’s acid bath. As it moves into the small intestine—where the pH is more neutral—the coating dissolves. A pressure-triggered membrane then releases its contents in sync with natural intestinal movements, ensuring that the therapeutic payload reaches its destination intact.
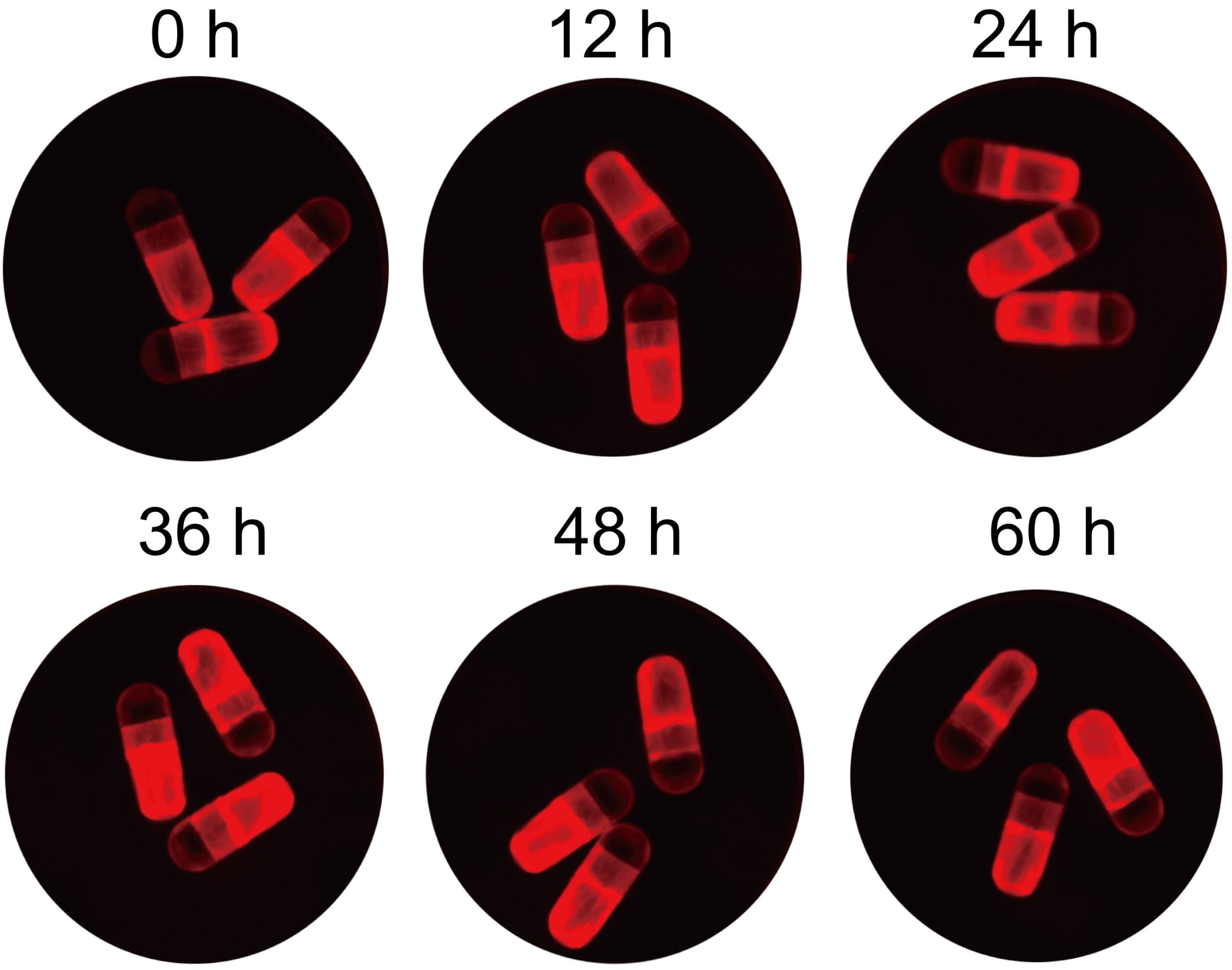
Inside, nanoparticles made from a specialized blend of G0-C14, PLGA, and PEG-lipid components protect the mRNA from degradation. This formulation is fine-tuned for mucus penetration and escape from endosomes—tiny cellular compartments that can otherwise trap and destroy the delivered message. Among several tested mixtures, a 5% DMPE-PEG composition proved the most effective at enabling gene expression in lab studies.
Teaching Intestinal Cells to Heal
The researchers tested RNACap in rats, focusing on interleukin-10 (IL-10), a naturally occurring anti-inflammatory molecule. By delivering IL-10 mRNA orally, they aimed to coax intestinal cells to produce this protective protein, potentially offering a treatment for inflammatory bowel diseases like Crohn’s disease and ulcerative colitis.
The results were striking. Once the capsule released its contents, intestinal cells successfully translated the mRNA into IL-10 protein, which appeared in both the blood and colon tissue. Levels of harmful proinflammatory cytokines dropped significantly. Rats with experimentally induced colitis showed improved symptoms and tissue health, whether the condition was in its early or more advanced stages.
Importantly, the therapy showed no detectable toxicity. Blood tests, cytokine panels, and microscopic examinations revealed no signs of harmful inflammation. Serum cytokine levels remained low, with only modest increases in IL-1Ra, IL-5, and IL-6—well within safe limits.
This isn’t just delivering a drug; it’s teaching the body to heal itself by programming its own cells.
From Rats to Swine: A Step Toward Humans
To gauge whether this technology could work in larger, human-like digestive systems, researchers tested RNACap in Yorkshire swine. Within 8.5 hours of oral administration, they detected measurable mRNA expression in intestinal tissues—an encouraging sign that the capsule’s design scales to larger organisms.
This swine model is critical because it bridges the gap between rodent experiments and human trials. Digestive physiology in pigs closely mirrors that of humans, meaning successful results here suggest feasibility in future clinical applications.
Breaking Free from Freezers and Needles
One of RNACap’s hidden advantages is its compatibility with liquid formulations, avoiding the cumbersome lyophilization (freeze-drying) process typically required to stabilize mRNA for storage. Lyophilization adds cost, reduces efficacy, and slows global distribution. By keeping the mRNA in liquid form, RNACap could simplify manufacturing, make distribution faster, and broaden access to cutting-edge therapies—especially in regions lacking specialized cold-chain infrastructure.
This innovation could be transformative during future pandemics, where rapid, widespread deployment of mRNA-based treatments or vaccines is crucial. Instead of lining up at clinics for injections, people might one day receive a bottle of capsules to take at home, dramatically accelerating public health responses.
Looking Ahead: A New Pill for a New Era
While RNACap has not yet been tested in humans, its success in rats and swine represents a paradigm shift. For conditions rooted in the gut—like inflammatory bowel diseases, certain cancers, and even metabolic disorders—oral mRNA therapeutics could offer targeted, non-invasive, self-administered treatments.
Even beyond gastrointestinal diseases, the ability to program intestinal cells opens possibilities for systemic therapies, where proteins produced locally could circulate throughout the body to treat autoimmune diseases or deliver vaccines.
Researchers caution that more work is needed to optimize shelf life, refine dosing, and ensure safety in humans. Clinical trials will determine whether this promising technology can truly revolutionize medicine.
But the vision is clear: a future where pills can deliver genetic instructions, turning cells into miniature factories that fight disease from within—without a single needle in sight.
Reference: Xiangang Huang et al, Oral delivery of liquid mRNA therapeutics by an engineered capsule for treatment of preclinical intestinal disease, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adu1493