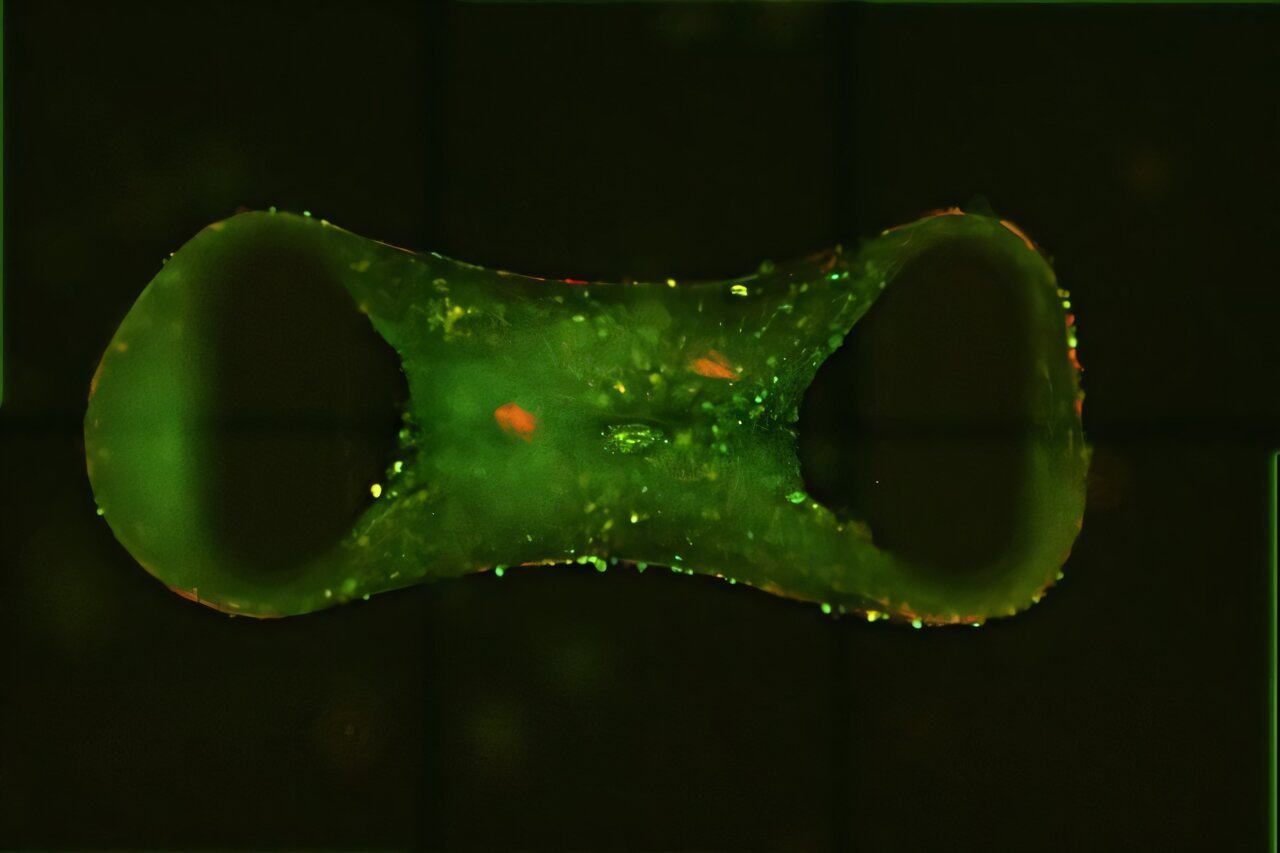
In a quiet laboratory, a few tiny structures no larger than chia seeds throb rhythmically under a microscope. They are not toys, nor simulations—they are living, beating human heart tissues. And they may be about to revolutionize the way we fight heart disease.
In a world-first breakthrough, scientists from QIMR Berghofer Medical Research Institute’s Cardiac Bioengineering Lab have created three-dimensional, lab-grown heart tissues that don’t just look like heart muscle—they act like it too. These “cardiac organoids,” derived from human stem cells, behave strikingly like adult human hearts, offering scientists an unprecedented new way to study disease and test treatments.
The research, published in Nature Cardiovascular Research, could accelerate the development of lifesaving therapies for some of the most devastating—and least understood—heart conditions, especially those affecting children and patients with rare genetic mutations.
From Stem Cells to Beating Hearts
At the heart of the innovation lies the use of human pluripotent stem cells—remarkable cells with the ability to transform into virtually any type of tissue in the human body. For years, scientists have tried to coax these cells into becoming mature heart cells to study cardiac diseases or test drugs. But there was a problem: these lab-grown heart cells typically stopped short of full maturity. They resembled fetal heart tissue, not the robust muscle of a fully developed human heart. This limitation made it difficult to model adult or childhood heart disease accurately.
The QIMR Berghofer team, led by Professor James Hudson, cracked the code by mimicking something the human heart responds to in life: exercise.
By activating two key biological pathways that mimic the effects of physical activity, the scientists succeeded in pushing the lab-grown tissues to behave more like genuine adult heart muscle. They didn’t just look like heart cells under the microscope—they pumped, contracted, and even responded to drugs in ways that mirror real human hearts.
“These cardiac organoids may be the size of a chia seed,” says Professor Hudson, “but they offer a platform to discover new treatments. There’s a huge benefit to studying heart diseases in this way—using human cardiac organoids allows us to screen many more compounds, speeding up the process of drug development.”
Recreating Disease—One Beat at a Time
The ability to mimic adult heart function in a petri dish is a breakthrough by itself. But what makes this research truly extraordinary is how scientists used these miniature hearts to recreate genetic heart diseases—including conditions that have long baffled researchers and doctors alike.
Among the most challenging diseases the team modeled was desmoplakin cardiomyopathy, a genetic disorder that disrupts the proteins which glue heart cells together. The condition causes scarring (fibrosis), weakens the heart’s ability to pump blood, and can lead to heart failure or sudden death. Studying this disease has traditionally been difficult because it’s rare, often appears in childhood, and lacks robust experimental models.
With the new cardiac organoids, that barrier fell.
By introducing specific genetic mutations—in genes like ryanodine, calsequestrin, and desmoplakin—the researchers were able to replicate the key hallmarks of the diseases in the lab-grown tissues. The miniature hearts didn’t just display the abnormalities at a molecular level; they began to behave like diseased hearts, losing pumping strength and developing fibrotic scars—just as they do in human patients.
Hope in a Petri Dish: A New Drug Shows Promise
Once the team had successfully engineered these disease models, they turned their attention to treatment. Among the therapies tested was a class of experimental compounds known as BET inhibitors—short for bromodomain and extra-terminal protein inhibitors. These drugs have been making waves in cancer research for their ability to switch off harmful genes, but their potential in heart disease had remained largely untapped.
The researchers treated the damaged cardiac organoids with one of these BET inhibitors—and the results were astonishing.
“In the cardiac organoids, the disease caused scarring and made the tissue pump less effectively—similar to what happens in patients’ heart disease,” explains Professor Hudson. “We then tested a new type of drug… and found this medication improved the heart tissue’s function.”
This marks one of the first demonstrations that lab-grown human heart tissue can not only model complex diseases but also be used to identify new therapeutic strategies with real potential to improve human lives.
A Collaborative Path Toward Healing Tiny Hearts
The project’s success was fueled by powerful collaboration between QIMR Berghofer, Murdoch Children’s Research Institute (MCRI), and The Royal Children’s Hospital in Melbourne. Using advanced genetic and protein analysis, MCRI researchers helped dissect the inner workings of these heart diseases and validate the findings using samples from the Melbourne Children’s Heart Tissue Bank—a rare and precious resource containing donated heart tissue from some of the most vulnerable young patients.
Associate Professor Richard Mills from MCRI underscores the importance of this partnership: “Our approach allows us to more accurately model childhood heart conditions to ultimately find better treatments for some of the sickest people in our community.”
He adds, “The collaboration with QIMR Berghofer and The Royal Children’s Hospital is accelerating progress towards these goals, and this approach has the potential to be used across a whole spectrum of childhood heart disease.”
The Future of Heart Disease Research Is Beating in the Lab
While these lab-grown hearts may be small, their impact is monumental. The implications extend far beyond rare childhood diseases. These cardiac organoids could soon become essential tools in screening drugs for heart safety, personalizing treatments based on a patient’s genetic profile, and even testing the effects of diet, toxins, or viral infections on the heart.
For families affected by congenital or genetic heart disease, the hope is tangible. With each beat of these tiny tissues, researchers get closer to understanding what goes wrong in the hearts of real patients—and how to fix it.
And for the scientific community, the achievement signals a major shift: from observing disease in the clinic to building it, testing it, and treating it in the lab—organ by organ, cell by cell, beat by beat.
Reference: Mark W. Pocock et al, Maturation of human cardiac organoids enables complex disease modeling and drug discovery, Nature Cardiovascular Research (2025). DOI: 10.1038/s44161-025-00669-3