For decades, scientists have known that the brain is a bustling universe of signals—tiny electrical impulses racing across vast networks of neurons. But what if one overlooked class of proteins holds the key to treating some of the most challenging psychiatric and neurological conditions of our time? A groundbreaking new study from Johns Hopkins Medicine suggests exactly that.
The Mysterious Proteins That Weren’t So Silent After All
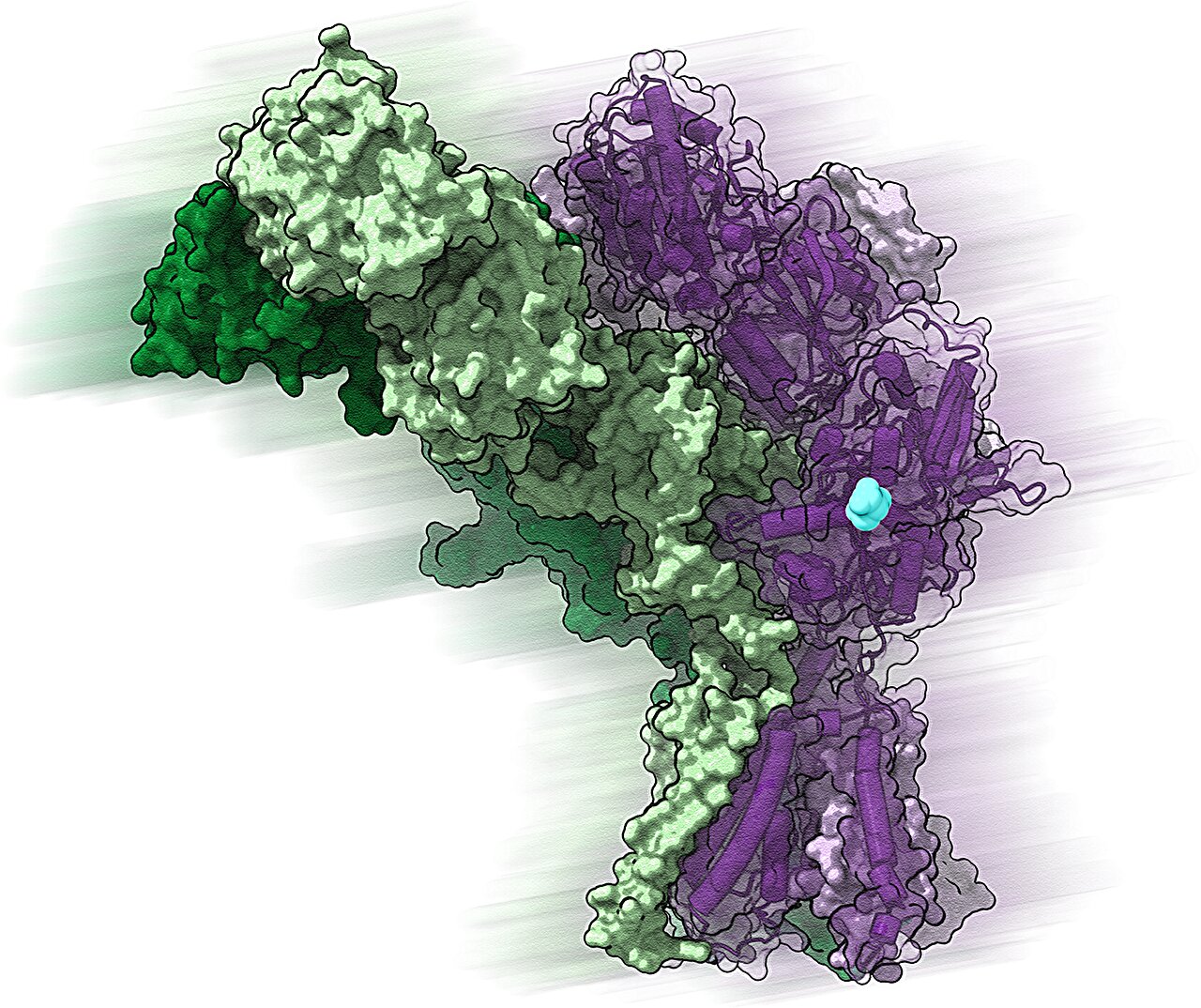
The focus of the research is a group of proteins known as delta-type ionotropic glutamate receptors, or GluDs. These proteins sit on the surface of brain cells, where they help regulate communication between neurons. For years, many researchers believed GluDs were mostly dormant, almost like sleeping guardians of the brain. But Edward Twomey, Ph.D., and his team at Johns Hopkins University School of Medicine have shown otherwise.
“Our findings indicate they are very much active and offer a potential channel to develop new therapies,” Twomey explains.
By using an advanced imaging technique called cryo-electron microscopy, the team captured high-resolution snapshots of GluDs, revealing their intricate structure. They found that each GluD protein contains a central ion channel—a tiny tunnel that charged particles pass through. This passageway allows GluDs to interact with neurotransmitters, the chemical messengers that carry information between brain cells. In essence, GluDs help shape how neurons talk to one another, making them fundamental to the creation and maintenance of synapses, the junctions where brain communication happens.
Why GluDs Matter for Mental Health and Movement
The discovery goes far beyond academic curiosity. Malfunctions in GluD proteins are thought to play a role in several psychiatric and neurological conditions. In schizophrenia, for instance, GluDs appear to be underactive, reducing the brain’s ability to form and maintain stable connections. On the other hand, in cerebellar ataxia—a disorder that impairs movement, balance, and sometimes memory—GluDs become hyperactive, firing signals even when they shouldn’t.
This duality offers a promising therapeutic opportunity. If GluDs can be “dialed up” in conditions like schizophrenia, or “dialed down” in disorders like cerebellar ataxia, scientists could create a new class of precision drugs aimed at restoring balance to the brain’s communication systems.
“Because GluDs directly regulate synapses, we could potentially develop a targeted drug for any condition where synapses malfunction,” Twomey says.
A Window Into the Future of Brain Medicine
The potential applications don’t stop there. Synapses are central to memory and learning, and their decline is often linked to aging and neurodegenerative conditions such as dementia. By preserving GluD function, future drugs could help protect memory and cognitive ability, offering hope for millions at risk of age-related decline.
The team’s findings also hint at new ways to tackle psychiatric disorders like anxiety, where GluD mutations may distort normal signaling patterns. A drug designed to fine-tune GluD activity could rebalance these systems, potentially easing symptoms without the broad and sometimes harsh effects of current treatments.
From Discovery to Therapy
Of course, the leap from laboratory discovery to an actual medication is a long one. Twomey and his colleagues are now looking to collaborate with pharmaceutical companies to transform their findings into viable therapies. Their immediate focus is on developing drugs that can specifically regulate GluD activity—quieting overactive receptors or awakening underactive ones depending on the disorder.
In parallel, the team is investigating genetic mutations in GluDs that are directly linked to conditions like schizophrenia and anxiety. By understanding how these mutations disrupt normal function, researchers hope to design therapies that address the root of the problem, not just its symptoms.
To protect their methods, Johns Hopkins University has already filed a patent covering the techniques used to measure electrical activity in GluDs. This move signals the institution’s confidence in the potential for real-world applications of this research.
A Turning Point in Neuroscience
The discovery of GluDs’ true activity marks a shift in how we think about brain proteins once considered passive. It also underscores a larger truth: the brain still holds countless secrets, and unlocking them may open doors to entirely new ways of treating disease.
For patients and families struggling with the challenges of psychiatric disorders, movement disorders, and memory loss, this research represents more than just a scientific breakthrough—it offers hope. Hope that one day, therapies built on the hidden power of GluDs could restore balance, sharpen memory, and ease the burdens of conditions that have long defied effective treatment.
As Twomey and his team continue their work, one thing is clear: the brain’s “silent” gatekeepers may soon become the centerpiece of a new era in neuroscience and medicine.
More information: Haobo Wang et al, Delta-type glutamate receptors are ligand-gated ion channels, Nature (2025). DOI: 10.1038/s41586-025-09610-x