In the endless search to heal the body without wounding the mind, scientists have often walked a fine line—between relief and side effects, between breakthrough and backlash. In the realm of obesity and type 2 diabetes, that line has been especially cruel. For millions of people around the world, treatments that offer real metabolic benefits often come with a bitter trade-off: constant nausea, vomiting, and a persistent sense of unease. Yet what if the answer was not in louder, more forceful therapies—but in the whisper of a signal, deep within the brainstem, emitted not by neurons, but by their silent, overlooked companions: glial cells?
Researchers at the University of Pennsylvania and Syracuse University have found a surprising, gentle key to this dilemma—a signaling peptide known as octadecaneuropeptide (ODN). Produced by glial cells in the hindbrain, this peptide does something astonishing: it suppresses appetite, improves glucose metabolism, and enhances insulin sensitivity—all without triggering nausea, vomiting, or cardiovascular side effects.
This revelation may be the dawn of a new era in metabolic medicine, one rooted not in the brute force of pharmacology but in the elegant rewiring of the brain’s natural language.
The Broken Compass of Metabolic Disease
To understand the significance of this discovery, we first have to grasp the fundamental betrayal that occurs in obesity and type 2 diabetes. These conditions are not simply a matter of eating too much sugar or failing to exercise. They are disorders of homeostasis, where the body’s most basic systems for regulating weight and blood sugar become misaligned—like a compass spinning aimlessly in a magnetic storm.
In obesity, the brain defends excess fat as though it were essential for survival. Hunger increases even as fat stores overflow, and energy expenditure plummets as if the body were facing famine. Similarly, in type 2 diabetes, the liver continues to pump out glucose despite dangerously high blood sugar levels, convinced that the body still needs fuel.
This is not mere resistance to insulin—it is resistance to reality. And it is the brain, particularly regions like the hypothalamus and the dorsal vagal complex (DVC) of the hindbrain, that orchestrates this delusion. These areas are designed to sense nutrient levels and adjust behavior and physiology accordingly. But in metabolic disease, these signals become corrupted, biased toward sustaining the very imbalances that need correction.
The Rise of Glial Intelligence
For decades, scientists focused almost entirely on neurons—the brain’s electrical dynamos—as the key drivers of hunger, satiety, and glucose regulation. But in recent years, a quiet revolution has taken place. Glial cells, once considered the brain’s support staff, have revealed themselves as powerful partners in metabolic control.
Among these, astrocytes—star-shaped cells that envelop synapses—respond to glucose deprivation and influence feeding behavior. Tanycytes, specialized cells lining the brain’s ventricles, detect sugars, amino acids, and lipids, and help shuttle hormones like insulin and GLP-1 receptor agonists into the brain.
Both astrocytes and tanycytes produce ODN, a peptide derived from a protein known as diazepam binding inhibitor (DBI). In the hypothalamus, ODN levels rise in response to feeding and glucose intake. Yet its role in the hindbrain—the DVC—remained a mystery. Until now.
A Peptide with a Purpose
In a landmark study published in Science Translational Medicine, researchers launched an ambitious investigation into the function of ODN in the DVC, aiming to determine whether this glial-derived peptide could act as a therapeutic switch for appetite, blood sugar, and hormonal balance.
They began with direct injections of ODN—and a more stable analog called tridecaneuropeptide (TDN)—into the brain’s fourth and lateral ventricles. What followed was nothing short of remarkable.
In both lean and obese rats, ODN reliably suppressed food intake. But in obese animals, the effect was more profound and sustained. These rats not only ate less, but their meal size and duration decreased, suggesting a recalibration of hunger itself. More than just skipping a snack, these animals appeared less driven to eat in a way that reflected genuine satiety—not aversion or discomfort.
Simultaneously, ODN enhanced glucose tolerance and insulin sensitivity, but without boosting insulin secretion—indicating improved tissue responsiveness to insulin rather than a compensatory overproduction. This is a critical distinction, as excessive insulin can itself drive weight gain and metabolic dysfunction.
Even when glucose levels were artificially lowered through experimental glucoprivation (using 5-thio-D-glucose or insulin), ODN still protected against the typical hunger surge and rise in blood glucose, blunting the counter-regulatory response. It lowered glucagon and free fatty acids—hormones that would normally spike to raise blood sugar—without affecting stress hormones like corticosterone. The effect was targeted, not traumatic.
No Nausea, No Panic, No Problem
The real surprise came when researchers tested whether ODN carried the common side effects of current therapies like GLP-1 receptor agonists. These drugs, while effective in treating obesity and diabetes, often cause nausea, vomiting, and gastrointestinal distress, limiting their tolerability in many patients.
Using musk shrews—a species capable of vomiting—they found that ODN did not trigger emesis, nor did it increase kaolin consumption, a behavior in rodents that signals nausea. Even when ODN’s pathway was blocked, the anorexic effects of GLP-1 agonists were only partially diminished, suggesting that ODN contributes to appetite suppression but not to sickness.
Furthermore, ODN had no measurable impact on physical activity, body temperature, or heart rate—key safety markers that often trip up central nervous system–acting agents.
Mapping the Molecular Landscape
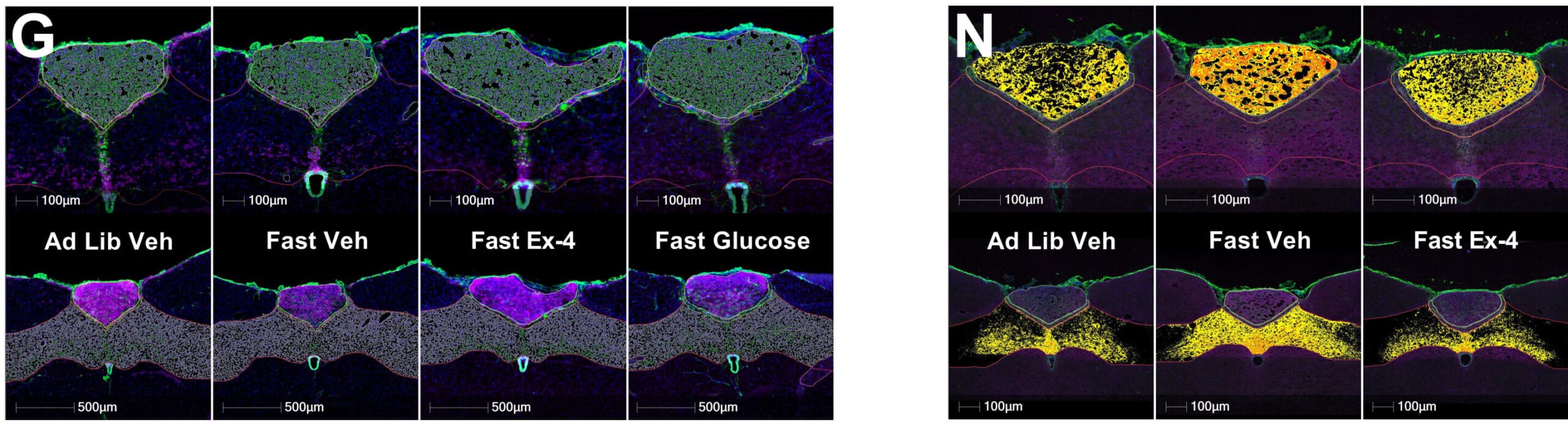
To better understand where and how ODN exerts its effects, the researchers turned to molecular imaging and transcriptomic datasets. Using immunofluorescence and in situ hybridization, they mapped DBI mRNA expression across the DVC, showing clear upregulation in response to refeeding and glucose administration, as well as exposure to GLP-1 receptor agonists.
Single-nucleus transcriptomics pinpointed DBI production to specific glial cell populations, revealing a glia-to-neuron signaling axis that had been hiding in plain sight. This axis doesn’t simply amplify neuronal commands—it may initiate them, setting the tone for appetite, hormone release, and even glucose production in the liver.
When this signaling was disrupted—either with antibodies or receptor antagonists—glucose control worsened and GLP-1–induced food suppression faltered. The implication was clear: ODN is not a passenger; it’s a driver.
A Shot at the Future: Peripheral ODN Analogs
Perhaps the most tantalizing finding came from trials with TDN, the synthetic, more stable cousin of ODN. In obese mice, daily peripheral (i.e., outside the brain) injections of TDN led to sustained reductions in food intake and a 4.7% weight loss in just nine days.
Using the gold standard of metabolic testing—the hyperinsulinemic-euglycemic clamp—scientists confirmed that TDN-treated mice required significantly more glucose to maintain normal blood sugar, a direct sign of improved insulin action. It also suppressed hepatic glucose production, reversing one of the core defects of type 2 diabetes.
TDN, unlike many drugs targeting the brain, accomplished all this without needing direct brain injection and without collateral damage to cardiovascular function or behavior. The door to a safe, scalable treatment had opened.
Toward a Gentler, Smarter Medicine
The discovery of ODN’s central role in appetite and glucose regulation isn’t just a win for science—it’s a shift in philosophy. It suggests that we don’t always need to override the brain’s systems to fix them. Sometimes, the better strategy is to remind the brain of what it already knows—to restore the natural flexibility that allows us to eat when we’re hungry, stop when we’re full, and keep blood sugar in a healthy range without panic.
Obesity and type 2 diabetes have long been framed as diseases of willpower, or worse, punishment for lifestyle. But these findings reinforce what science has begun to understand more deeply: these are diseases of dysregulated sensing, of neural systems that have lost their ability to adjust. The brain, like the body, sometimes forgets how to listen.
With the discovery of ODN’s function, researchers have identified a new channel through which we can whisper—not shout—our instructions. This channel doesn’t bludgeon the brain with sickness to enforce compliance. It aligns the internal compass, gently, toward balance.
The Road Ahead
Though the findings are profound, this is still early science. Human trials will be essential. The path from a mouse to a medicine cabinet is long and littered with failures. Yet the elegance of ODN—its safety, specificity, and synergy with existing pathways—makes it a uniquely promising candidate.
The notion that glial cells, long considered the quiet custodians of the brain, could hold the key to curing some of our most intractable metabolic disorders is poetic. They have waited in the shadows, watching neurons take the spotlight. Now, at last, they are ready to speak—and the body, it seems, is ready to listen.
Reference: Caroline E. Geisler et al, Hindbrain octadecaneuropeptide gliotransmission as a therapeutic target for energy balance control without nausea or emesis, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adu6764