Imagine struggling with a loss of motivation so deep that even your favorite foods lose their spark, or living with anxiety so intense that every unfamiliar sound feels like a looming threat. These are realities for millions of people living with depression and anxiety. Scientists have long searched for the roots of these conditions within the brain, but finding causal mechanisms—clear evidence that one brain region drives these feelings—has been remarkably difficult.
Now, researchers at the University of Cambridge have taken a bold step forward. By studying marmosets, small monkeys whose brains share important similarities with ours, they have uncovered how a key region of the prefrontal cortex—area 46 of the dorsolateral prefrontal cortex (dlPFC)—influences both motivation and threat responses. Their findings, published in Science, illuminate not only the architecture of emotion in the brain but also how treatments like ketamine might help restore balance in patients with treatment-resistant depression.
Why the Dorsolateral Prefrontal Cortex Matters
The dlPFC is often described as one of the most “human” parts of the brain. It is essential for higher-order functions: focusing attention, juggling multiple thoughts in working memory, inhibiting impulses, and imagining the future. Yet its role extends beyond abstract cognition. For years, neuroimaging studies have shown that the dlPFC is active during emotional regulation—when people reframe stressful events or suppress fear.
Importantly, the dlPFC is also a therapeutic target. Noninvasive brain stimulation, such as transcranial magnetic stimulation (TMS), can activate the dlPFC and has been shown to relieve depressive symptoms, especially when conventional antidepressants fail. Still, much of the evidence has been correlational. Scientists knew the dlPFC was involved, but not precisely how it causally shaped motivation or fear.
That is the puzzle the Cambridge researchers set out to solve.
The Marmoset as a Model
To answer such complex questions, the team turned to marmosets, small primates whose brains share structural and functional parallels with humans. Six marmosets participated in motivation experiments and pathway-specific manipulations, while seven others were tested for hemispheric asymmetry—differences between the left and right sides of the brain.
The researchers used cutting-edge chemogenetic and pharmacological techniques to selectively “switch off” area 46. This allowed them to ask a simple but profound question: what happens to motivation and fear when this key prefrontal region is silenced?
Testing Motivation and Fear
The team designed clever behavioral tasks to measure two core dimensions of emotional life: the drive to pursue rewards and the reactivity to threats.
Motivation was assessed using a progressive ratio task. In this setup, marmosets had to tap a touchscreen more and more times to earn drops of milkshake—a treat they usually find irresistible. A second measure, the sucrose preference test, checked whether the animals’ capacity to enjoy sweetness was intact.
Threat reactivity, by contrast, was measured using the “human intruder” test. A person the marmosets did not know entered the room and stared at them. How the animals reacted—whether with cautious curiosity or with signs of heightened anxiety—served as an index of their emotional state.
The Motivational Deficit: When Effort Loses Its Worth
Inactivation of area 46 produced a striking effect. The marmosets became less willing to work for the milkshake reward. They made fewer responses and earned fewer rewards. Yet their sucrose preference remained unchanged. In other words, they still liked sweet things—they just lost the motivation to put in effort to get them.
This mirrors a symptom often seen in depression known as anhedonia, where patients report not so much a loss of pleasure itself but a loss of drive to pursue pleasurable activities. The Cambridge study suggests that dysfunction in dlPFC area 46 can directly trigger this kind of motivational deficit.
Anxiety Amplified: When Threats Loom Larger
The story did not end with motivation. When area 46 was inactivated, the marmosets also displayed heightened reactivity to the human intruder. They were more anxious, more cautious, and more defensive. This indicates that area 46 normally plays a role in damping down excessive threat responses, keeping fear in check.
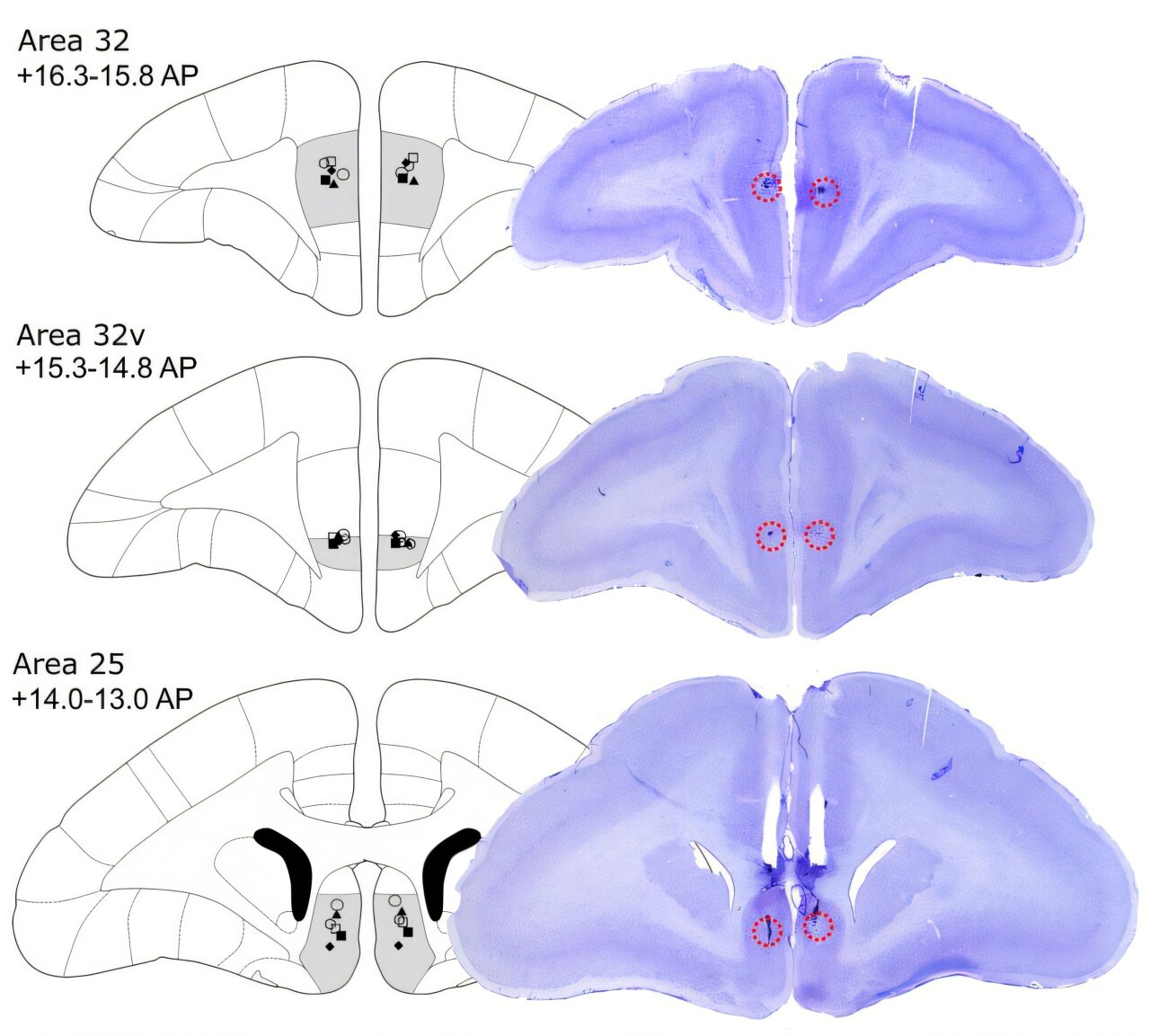
Intriguingly, these two effects—on motivation and on threat reactivity—were mediated by distinct pathways. Projections from area 46 to dorsal area 32 were critical for motivation, while projections to ventral area 32 (A32v) influenced threat reactivity. This fine-grained mapping reveals that the prefrontal cortex does not simply act as a “brake” on emotion, but rather orchestrates different emotional processes through specific circuits.
The Left Hemisphere Holds the Key
The study also uncovered a striking asymmetry between the brain’s hemispheres. Inactivating the left dlPFC increased threat reactivity, while silencing the right had no measurable effect. This finding resonates with clinical observations: stimulation of the left dlPFC is associated with antidepressant responses in patients, while right-sided activation is more tied to inhibitory control.
By showing causal hemispheric differences in a primate model, the Cambridge team provided critical evidence that the lateralization seen in humans is not coincidental but rooted in fundamental brain organization.
Ketamine: Restoring Motivation Through Area 25
The researchers also tested how ketamine, a fast-acting antidepressant, interacted with these circuits. When area 46 was inactivated, the marmosets’ motivation plummeted. But administering ketamine—either systemically or directly into area 25, a region deep in the prefrontal cortex—reversed this deficit.
This finding is especially powerful because clinical studies in humans have repeatedly shown that the therapeutic response to brain stimulation is linked to activity in area 25, also called the subcallosal cingulate cortex. By bridging animal models with human clinical evidence, the Cambridge team demonstrated a mechanistic link between dlPFC dysfunction, area 25 activity, and the restorative effects of ketamine.
Building a Map of Emotional Regulation
Taken together, the results paint a compelling picture. Area 46 of the dlPFC forms part of a network with areas 32 and 25, orchestrating both positive and negative aspects of emotional behavior. Silencing area 46 produces a dual syndrome: diminished motivation, reminiscent of depression, and heightened threat reactivity, reminiscent of anxiety. These effects, though, are mediated by distinct pathways and can be selectively modulated by treatments like ketamine.
For the first time, scientists have causal evidence that asymmetric prefrontal networks regulate emotion in primates in ways that closely resemble human clinical findings.
Why This Matters for Mental Health
Depression and anxiety remain among the most common and debilitating mental health conditions worldwide. For many patients, current treatments are insufficient. Understanding the neural circuits that drive these disorders is therefore essential for designing more effective therapies.
The Cambridge study provides a roadmap. By showing how dlPFC networks with area 32 and area 25 regulate motivation and fear, it highlights potential targets for both brain stimulation and pharmacological intervention. It also strengthens the rationale for therapies that focus specifically on the left dlPFC, which appears to hold a privileged role in mood regulation.
Most importantly, these findings give hope. They suggest that the seemingly intractable symptoms of depression and anxiety—lack of motivation, excessive fear—are not just vague feelings but the product of identifiable circuits. And if circuits can be mapped, they can be modulated, offering patients a clearer path to recovery.
More information: Christian M. Wood et al, Dysfunction in primate dorsolateral prefrontal area 46 affects motivation and anxiety, Science (2025). DOI: 10.1126/science.adx4142
Michael T. Treadway, A shared circuit might link depression and anxiety, Science (2025). DOI: 10.1126/science.aea0913