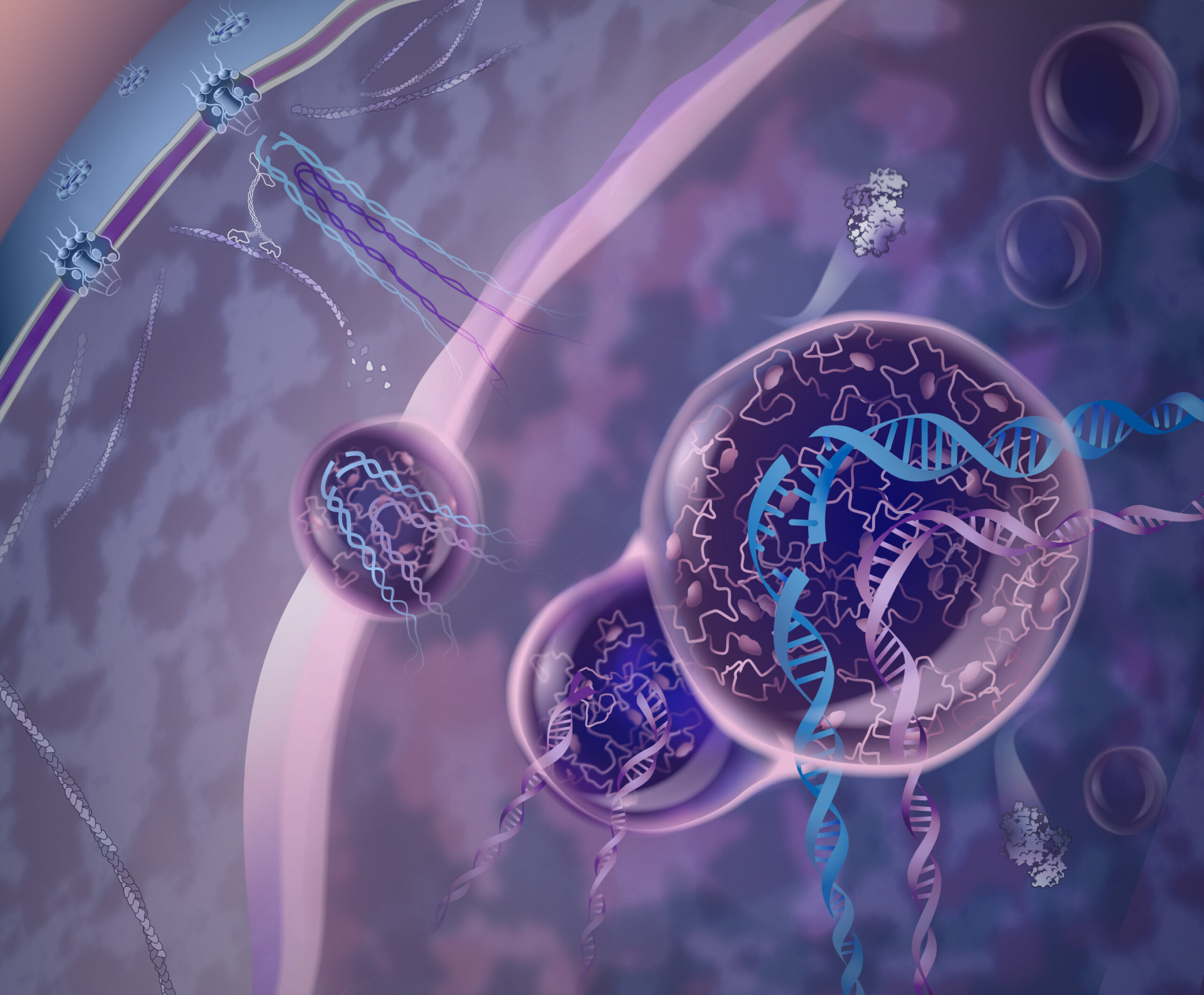
In the intricate world of cellular processes, DNA repair is a crucial mechanism that keeps the genetic blueprint intact. When DNA breaks inside a cell, it can set off a chain reaction of errors that, if left unchecked, could lead to diseases like cancer. Traditionally, DNA repair has been understood as a process dependent on various proteins and enzymes working in harmony to restore damaged strands. However, a breakthrough discovery by scientists Irene Chiolo and Chiara Merigliano at the USC Dornsife College of Letters, Arts and Sciences has added a fascinating layer to this intricate system.
Their research, published in Molecular Cell, reveals that a protein known as Nup98, long recognized for its role in regulating the movement of molecules in and out of the cell’s nucleus, plays a surprising secondary role in DNA repair—particularly in areas where damage is most dangerous. Nup98 forms protective condensates, bubble-like structures that act as shields around damaged DNA, reducing the risk of genetic mistakes and, ultimately, cancer. This discovery holds immense implications for cancer research and potential therapeutic interventions.
Nup98’s Dual Role in the Cell
Nup98’s primary function has long been to help regulate the flow of molecules between the cell’s nucleus and cytoplasm. It works as part of a larger structure known as the nuclear pore complex (NPC), which acts as a gatekeeper, controlling the movement of RNA and proteins. However, the new findings by Chiolo and Merigliano shed light on an unexpected role that Nup98 plays within the nucleus, particularly when DNA damage occurs in regions of the genome that are notoriously difficult to repair.
DNA is the blueprint of life, and any damage to it—whether caused by external factors like UV radiation or internal processes like oxidative stress—can lead to serious consequences. The genome contains both euchromatin, which is loosely packed and accessible for repair, and heterochromatin, which is densely packed and difficult to navigate. Heterochromatin, in particular, poses a significant challenge for DNA repair mechanisms. It contains repeated DNA sequences that can lead to errors during the repair process, making it much easier for the cell to misinterpret one stretch of DNA for another.
This is where Nup98 comes in. When DNA breaks occur in heterochromatin regions, Nup98 forms droplet-like condensates around the damaged DNA. These droplets create a protective bubble that shields the damaged strands and moves them out of the dense, packed area of heterochromatin. By doing so, Nup98 helps to prevent genetic mix-ups, reducing the likelihood of errors during repair that could lead to harmful mutations, such as those seen in cancer.
Nup98 and the Mobilization of Damaged DNA
Another remarkable aspect of Nup98’s role in DNA repair is its ability to mobilize damaged DNA to a safer area within the nucleus. Heterochromatin is tightly packed, making it an inhospitable environment for repair enzymes. If a damaged piece of DNA is left in this region, repair can be inaccurate, potentially causing genomic instability. Nup98 helps by temporarily lifting the damaged DNA out of the crowded heterochromatin and relocating it to a less crowded, more accessible area within the nucleus. This relocation is essential because it provides the repair machinery with a better environment to work, ensuring a more accurate repair process.
This movement of damaged DNA is not random, however. It’s a carefully coordinated process, and Nup98 plays a critical role in managing the timing of repair. In a highly controlled manner, Nup98 determines when certain repair proteins can come into play. This is particularly important because some proteins, like Rad51, can cause problems if they arrive too early in the repair process. If Rad51 is involved too soon, it could stitch together the wrong pieces of DNA, leading to potentially disastrous outcomes.
To prevent this from happening, Nup98’s condensates act as a temporary shield, keeping Rad51 and other repair proteins at bay until the time is right. Once the damaged DNA has moved into a more accessible area, and other repair mechanisms have set the stage, Nup98 allows Rad51 to take over and complete the repair. This careful coordination ensures that the repair process is as accurate as possible, minimizing the risk of harmful genetic rearrangements.
Implications for Cancer Research
The discovery of Nup98’s role in DNA repair has far-reaching implications for cancer research. DNA damage and genomic instability are well-known contributors to cancer development. When DNA is not repaired properly, mutations can accumulate over time, eventually leading to the uncontrolled cell growth that characterizes cancer.
Interestingly, Nup98 itself has been linked to cancer, particularly a form of leukemia known as acute myeloid leukemia (AML). Mutations in Nup98 have been shown to play a role in the development of this disease. By understanding how Nup98 functions in the repair process, scientists hope to gain insight into why these mutations are so dangerous and how they might be targeted for therapeutic purposes.
For example, in cancers like AML, where Nup98 mutations are common, there may be a potential opportunity to develop treatments that either specifically disrupt the mutated protein or target the cells carrying the mutation. Such targeted therapies could help to stop cancer cells in their tracks, potentially offering more effective and less harmful treatment options than traditional therapies.
The research also opens the door to exploring therapies that could enhance or mimic Nup98’s protective functions. By boosting the protein’s ability to shield DNA during the repair process, scientists could develop treatments that reduce the risk of genome instability—a major contributor to not just cancer, but also aging and other disorders related to genomic instability.
A Promising Avenue for Future Therapies
While much of the research conducted by Chiolo and Merigliano focused on fruit flies, their findings have broader implications for human health. Many of the DNA repair mechanisms in fruit flies are shared across species, making the fly an invaluable model for studying genome stability. The principles that govern DNA repair in fruit flies likely hold true in humans, providing a stepping stone for understanding similar processes in human cells.
Looking ahead, this discovery could lead to the development of new therapeutic strategies aimed at improving DNA repair mechanisms or specifically targeting the repair functions of Nup98. By enhancing the protein’s ability to repair broken DNA accurately, it may be possible to prevent the genomic instability that drives not only cancer but also aging and other genetic disorders.
Moreover, understanding the timing and coordination of Nup98’s role in DNA repair could lead to a better grasp of how other proteins involved in repair work. This could allow researchers to fine-tune therapeutic interventions and optimize the use of repair proteins in a clinical setting.
A Global Effort
The study conducted by Chiolo, Merigliano, and their collaborators is a remarkable example of international scientific collaboration. With 17 scientists from seven institutions contributing to the research, this study underscores the importance of teamwork in unraveling the complexities of biological systems. The collaborative nature of the study not only accelerated the research process but also brought together diverse expertise from different fields, providing a more comprehensive understanding of Nup98’s role in DNA repair.
By expanding our understanding of how cells repair their DNA, especially in regions that are difficult to navigate, this research lays the groundwork for future breakthroughs in cancer treatment and genome stability. The discovery of Nup98’s dual role highlights just how intricately connected cellular functions are and how even well-known proteins can have unexpected and life-saving roles in maintaining the integrity of the genome.
Conclusion: Protecting the Blueprint of Life
DNA is the blueprint of life, and ensuring its integrity is essential for health and survival. The findings of Irene Chiolo and Chiara Merigliano offer a new layer of understanding in the complex world of DNA repair. Nup98’s role as a guardian of the genome, helping to shield broken DNA and guide its repair, is a testament to the cell’s remarkable ability to maintain its stability in the face of damage.
As scientists continue to unravel the mysteries of DNA repair, the role of Nup98 provides hope for more targeted therapies to treat cancer, reduce aging-related genetic instability, and address other disorders tied to genome integrity. By understanding how the cell coordinates DNA repair, researchers are one step closer to unlocking the secrets that could one day revolutionize medicine and protect the genome for generations to come.
Reference: Chiara Merigliano et al, Off-pore Nup98 condensates mobilize heterochromatic breaks and exclude Rad51, Molecular Cell (2025). DOI: 10.1016/j.molcel.2025.05.012