In every human cell, an invisible war rages on. DNA—the delicate molecule that holds the code of life—is under constant attack. From ultraviolet rays to toxic chemicals, and even the body’s own metabolic byproducts, countless threats conspire to damage our genetic blueprint every day. The toll is staggering: up to 100,000 DNA lesions per cell, per day.
Among these assaults, one stands out as especially dangerous: the DNA double-strand break (DSB). When both strands of the DNA helix are snapped, the cell is left on the brink of catastrophe. If not swiftly repaired, a single DSB can trigger cell death or lead to mutations that pave the way for cancer, premature aging, immune disorders, and neurodegeneration.
To counter this threat, evolution has armed cells with an intricate defense: the DNA damage response (DDR)—a finely tuned orchestration of detection, signaling, and repair. Yet, even after decades of research, major parts of this process remain shrouded in mystery—especially the later stages where DNA ends are processed for repair. Now, a new study may have cracked open part of that black box.
Published in Nature Cell Biology, a groundbreaking investigation led by researchers at Boston University Chobanian & Avedisian School of Medicine, Massachusetts General Hospital, and Harvard Medical School has identified a previously overlooked gene, ZNF280A, as a vital player in DNA repair. And their discovery doesn’t stop there: it may also explain the troubling clinical features of a rare developmental disorder called 22q11.2 distal deletion syndrome.
From Silent Gene to Genetic Guardian
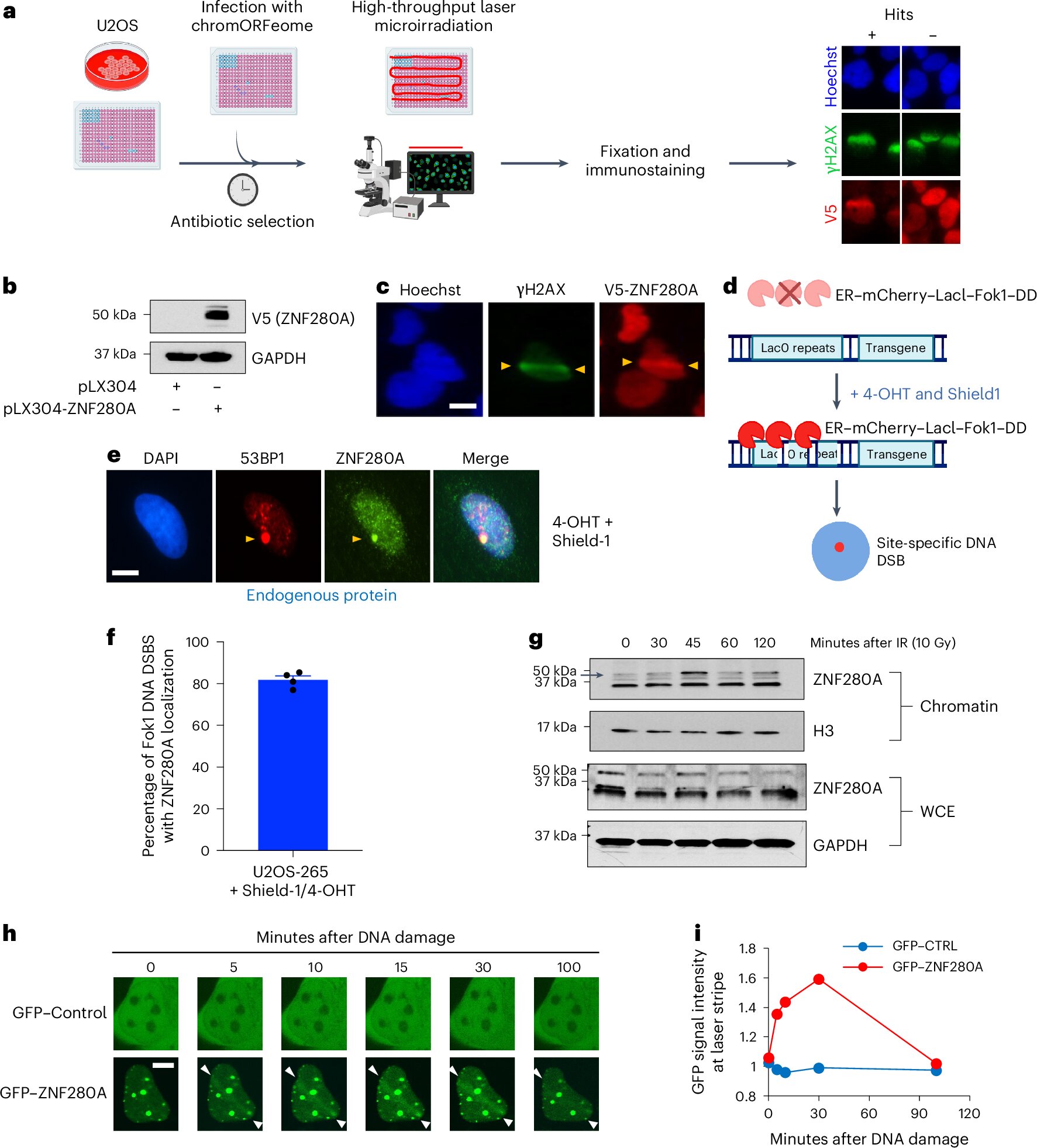
ZNF280A wasn’t on most scientists’ radar. A chromatin-associated protein—part of the complex molecular machinery that regulates gene expression—ZNF280A had no known role in DNA repair. That changed when the research team deployed a novel screening approach, developed in the lab of Dr. Raul Mostoslavsky at Massachusetts General Hospital, to track which chromatin factors rally to sites of DNA damage.
This strategy used high-throughput DNA open reading frame (ORF) sequences—an innovative method for bypassing the limitations of older screening tools like siRNA or CRISPR, which often miss essential genes whose deletion causes cells to die. ZNF280A stood out in the screens.
Multiple assays confirmed what the scientists suspected: ZNF280A was essential for repairing DNA double-strand breaks. But the real shock came when the researchers linked the gene to a specific group of patients with a missing piece of chromosome 22.
A Missing Gene, a Missing Answer
In some children born with 22q11.2 distal deletion syndrome, one copy of the ZNF280A gene is missing—a condition known as hemizygous deletion. These patients often struggle with serious medical issues: immune deficiencies, growth delays, microcephaly (abnormally small head size), and cognitive impairments.
For years, the cause behind this constellation of symptoms remained unclear. But when Dr. Mostoslavsky and co-corresponding author Dr. Thomas Clarke teamed up with pediatric clinicians from the Children’s Hospital of Philadelphia—renowned experts in this syndrome—they began to connect the dots.
Patient-derived cell lines with the specific deletion that includes ZNF280A were studied. The results were striking: these cells showed increased DNA damage and impaired ability to repair DSBs. When researchers reintroduced the ZNF280A gene into the cells, the DNA repair defects were partially reversed.
“It was a eureka moment,” Dr. Mostoslavsky said. “We realized that these children weren’t just missing a piece of DNA—they were missing a vital line of defense against genomic instability. That missing gene, ZNF280A, could explain many of the symptoms they experience.”
Genomic Instability: The Hidden Engine of Disease
DNA damage may seem like an abstract threat, but the consequences of unrepaired breaks are deeply personal. In 22q11.2 distal deletion syndrome, children often face developmental hurdles that reshape every aspect of their lives—from their immune systems to their cognitive growth. This new research suggests that defective DNA repair may be the common thread.
The clinical features seen in these patients eerily echo those observed in other disorders caused by mutations in known DNA repair genes. Until now, ZNF280A hadn’t been considered part of that group. But this discovery places it squarely at the heart of one of the most important biological processes: the preservation of genetic integrity.
And it doesn’t stop at rare disorders.
“Understanding how ZNF280A functions and how it’s regulated is essential not only for developmental syndromes but also for cancer biology,” Dr. Clarke noted. “Genomic instability is a hallmark of cancer. If we can manipulate the repair pathways that ZNF280A is involved in, we might one day develop targeted therapies.”
The Future of DNA Repair Research
This study opens several doors for future exploration. Scientists are now looking to uncover how ZNF280A is itself regulated—what switches it on or off, and how it interacts with other components of the DNA repair machinery. These insights could have ripple effects across multiple fields of medicine, from oncology to immunology and beyond.
Moreover, the team’s high-throughput screening method could pave the way for identifying other hidden repair factors, potentially rewriting what we know about the DDR.
“We’re just scratching the surface,” Dr. Mostoslavsky said. “There are likely dozens, maybe hundreds, of chromatin factors out there influencing DNA repair in subtle but powerful ways. We finally have a tool to find them.”
A New Era in Personalized Genomic Medicine
The implications of this work are vast. In the future, genomic testing might not just identify missing genes, but assess how those deletions influence a person’s DNA repair capacity—allowing for more personalized medical care for patients with syndromes like 22q11.2 distal deletion. It might also offer early warnings for cancer susceptibility or help tailor treatments that exploit tumor cell vulnerabilities in DNA repair.
For now, ZNF280A represents more than a gene—it represents a window into how life protects itself at its most fragile core. And for families grappling with the uncertainty of developmental syndromes, it offers something else: a clearer explanation, and perhaps one day, a path to intervention.
In a world where a single gene can alter the course of a life, this research reminds us just how powerful—and how fragile—our genetic machinery truly is. Every strand of DNA carries a story, and with each scientific breakthrough, we read it a little more clearly.
Reference: Thomas L. Clarke et al, ZNF280A links DNA double-strand break repair to human 22q11.2 distal deletion syndrome, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01674-1