In a world where bacterial infections are becoming harder and harder to treat, scientists at the University of Nottingham have stumbled upon a remarkable solution—not in a new drug, but in the shape of plastic itself.
Imagine a battlefield so small it fits on the tip of a catheter. Instead of chemical weapons, this battlefield uses geometry—tiny ridges and crevices—to confuse, trap, and disable bacteria before they can dig in and cause harm. This isn’t science fiction; it’s the reality of a groundbreaking study just published in Nature Communications.
The discovery, led by Professor Paul Williams and Professor Morgan Alexander, could dramatically change the way hospitals defend against some of their most persistent and deadly enemies: infections on medical devices. Catheters, breathing tubes, and other implants—often made of plastic—are breeding grounds for bacterial biofilms, which are slimy, shielded colonies that resist antibiotics and evade the body’s immune defenses. Once established, these biofilms are notoriously difficult to treat.
But what if you could stop bacteria before they ever get the chance to build?
Geometry as a Weapon Against Disease
The Nottingham research team did just that. Instead of relying on chemical warfare—like impregnating plastics with antibiotics, which risks fueling antibiotic resistance—they took a different path: physical design.
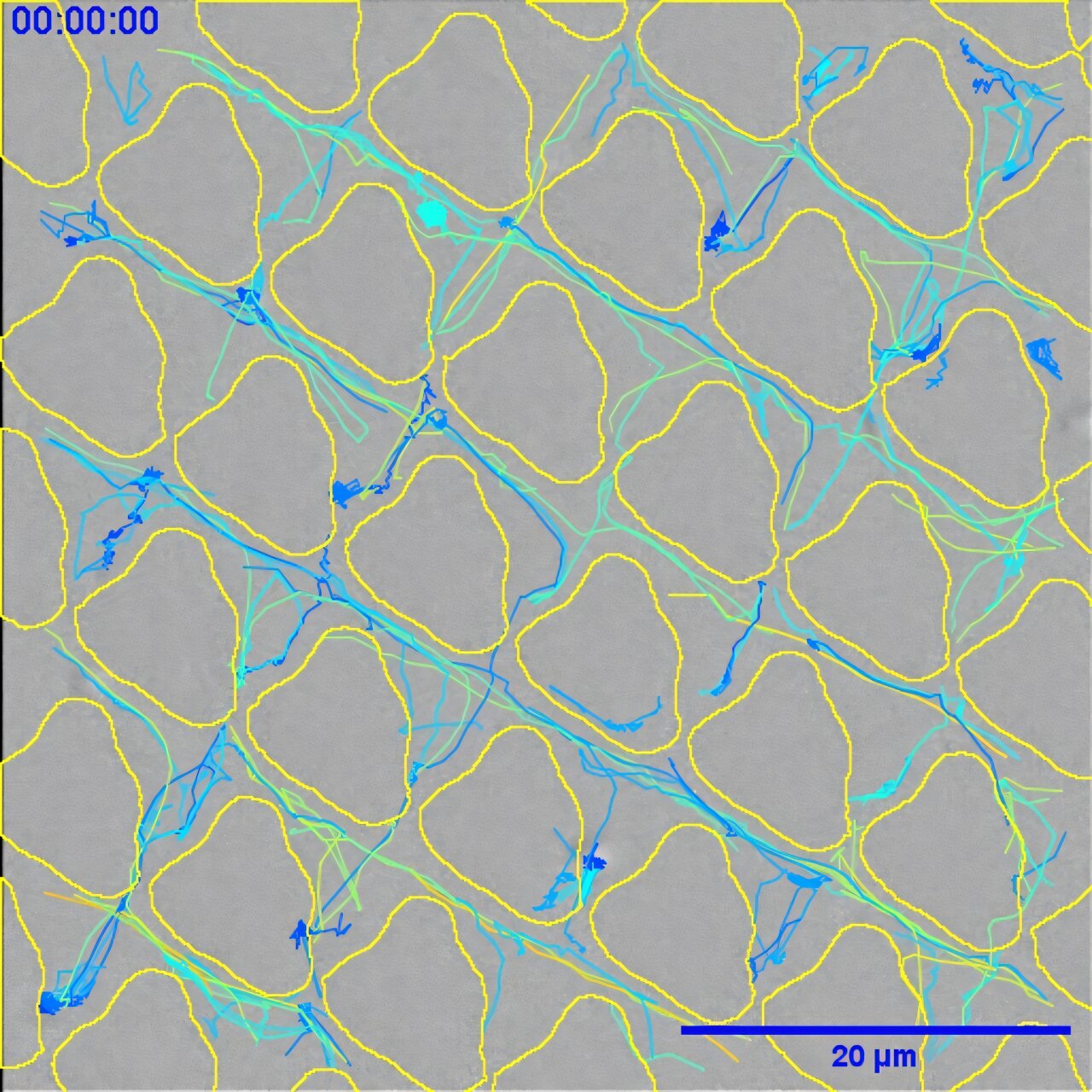
Working with collaborators from the School of Computer Science and a team in the Netherlands, the researchers used advanced screening techniques and machine learning to test over 2,000 microscopic surface patterns molded into different plastics. One material, polyurethane, commonly used in catheters, became the perfect testbed.
They discovered that certain tiny patterns could drastically reduce a bacterium’s ability to settle, grow, and form protective biofilms. How? By tricking bacteria into sabotaging themselves.
“On the best patterns, small crevices in the surface would trap the bacteria just long enough for them to release a kind of lubricant,” explained Professor Williams. “This slime-like substance actually stops them from sticking properly. They lose their grip—and their ability to form a biofilm.”
This subtle manipulation of bacterial behavior doesn’t just block their advance—it also seems to give the immune system a helping hand. With no protective fortress to hide behind, the bacteria are left exposed, vulnerable, and easier to eliminate by the body’s natural defenses.
A Breakthrough Without Antibiotics
The implications are enormous. For decades, efforts to prevent medical device infections have leaned heavily on chemical solutions—adding antibiotics or other antimicrobial agents to plastic surfaces. But this approach carries serious risks. Overuse of antibiotics in any form can lead to resistance, rendering those life-saving drugs useless in future battles.
“Our study took a different route,” said Professor Williams, a molecular microbiologist with years of experience in understanding bacterial behavior. “We wanted to create a landscape—made of the same plastic—that bacteria simply didn’t like. A surface that disrupts their ability to grow, without ever using a single molecule of antibiotic.”
Using artificial intelligence to process the vast dataset of surface designs and bacterial responses, the team identified not only what worked best but also why it worked. The designs that performed best were those that subtly manipulated the bacteria’s own survival instincts—causing them to produce lubricants that ironically prevented them from adhering to the surface they had just landed on.
Professor Alexander, an expert in polymer surfaces and cell interactions, emphasized another important advantage. “Patterned surfaces can be applied to existing device materials. That’s a huge win,” he said. “Instead of developing a new material from scratch, we can apply these designs to what’s already used in hospitals. That reduces costs, speeds up implementation, and makes commercialization far more feasible.”
Healing Without Harm
Biofilms are a major health hazard in medical settings. Infections related to implanted devices are common and dangerous, often requiring long-term antibiotic treatment or even surgical removal of the device. According to NHS reports, catheter-associated infections alone affect thousands of patients each year, leading to longer hospital stays and serious complications.
The study’s findings offer a way to break this cycle. By creating plastic surfaces that don’t just passively resist bacteria—but actively disarm them—hospitals could prevent many infections before they start.
“It’s proactive protection,” Williams said. “Not only are we making it harder for bacteria to stick, but we’re essentially waking up the immune system, allowing it to mop up the few cells that remain.”
The beauty of this approach is its simplicity. There’s no drug to degrade over time, no chemicals to wear off, no extra coatings to apply. It’s just design. And once that design is stamped into the plastic, it stays—working quietly, invisibly, every second the device is in use.
A Glimpse Into the Future of Infection Prevention
This discovery sits at the intersection of microbiology, materials science, and machine learning—a perfect storm of disciplines that, together, are transforming how we think about health care.
It’s a reminder that sometimes, the smartest solutions don’t require more force—they require more finesse. Instead of killing bacteria with stronger drugs, we can now confuse them with clever designs. Instead of dousing plastics with chemicals, we can reshape them to be inherently resistant to infection.
More importantly, this strategy holds up in the face of rising antibiotic resistance, one of the biggest threats to global health in the 21st century. It offers a future where we don’t have to rely on dwindling drug options but can instead use design principles baked into the very tools of medicine.
For patients, this could mean fewer infections. For hospitals, it could mean shorter stays, lower costs, and fewer complications. For the NHS and other health systems worldwide, it could mean saving millions—not just in pounds or dollars, but in lives.
And it all starts with a plastic surface, so small you’d need a microscope to see the pattern. But in that tiny design lies the power to stop infections before they ever begin.
Reference: Combinational discovery of micro topographical landscapes that resist biofilm formation through quorum sensing mediated autolubrication, Nature Communications (2025). DOI: 10.1038/s41467-025-60567