In the bustling world of plant cells, an unexpected discovery has just turned everything we thought we knew about epigenetics on its head. For years, scientists have understood that epigenetic tags—the chemical marks that regulate which genes are turned on or off—were primarily guided by other epigenetic modifications. But now, thanks to a breakthrough at the Salk Institute, we know that DNA itself may hold the power to regulate its own epigenetic changes.
The question that has puzzled biologists for decades was simple but profound: if epigenetics controls how genes are expressed, what controls epigenetics itself? What mechanism regulates the placement of those chemical tags that dictate cell function and identity? With this discovery, scientists have uncovered a novel way that genetic information can directly influence epigenetic changes, opening up exciting new possibilities for how we might control and manipulate these processes.
A Revolutionary Discovery in Plant Cells
To unravel this mystery, the Salk team turned to the plant model Arabidopsis thaliana, a small flowering weed that has long been a laboratory staple. While the plant may seem humble, its genetic simplicity makes it the perfect subject for complex studies. The researchers were particularly interested in how DNA methylation—a key epigenetic mark—was being applied in plant cells.
In plants, DNA methylation is a process where a small chemical group, called a methyl group, is added to certain parts of the DNA. This marks the DNA to be “turned off” or silenced, preventing the expression of specific genes. This process is crucial for regulating gene activity, controlling everything from plant development to responses to environmental changes.
But the mystery that intrigued the researchers was how the cell decides where to apply these methylation marks in the first place. Prior to this study, scientists understood how existing methylation patterns could be reinforced, but no one knew exactly how new patterns of DNA methylation were created during development or in response to stress.
How DNA Itself Can Set the Stage
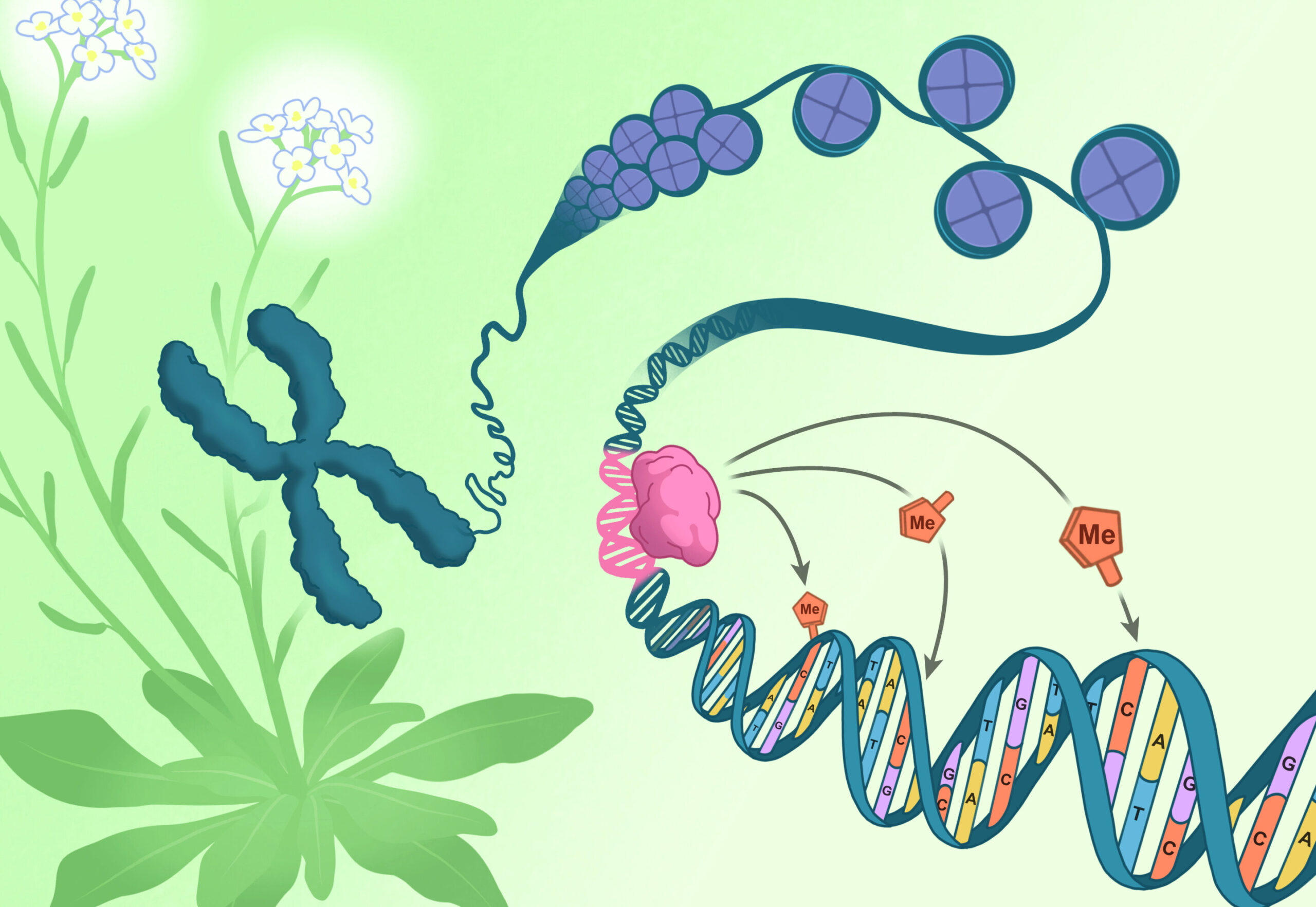
The breakthrough came when the team identified a surprising clue in Arabidopsis’s reproductive tissues. The researchers discovered a new mode of DNA methylation targeting that relied not on pre-existing epigenetic marks, but on specific DNA sequences. This was a major paradigm shift, as it upended the prevailing theory that DNA methylation was always directed by other epigenetic features.
“We wanted to know what was regulating epigenetic pathways to create new DNA methylation patterns during plant development, regeneration, and reproduction,” says Guanghui Xu, Ph.D., the study’s first author. “What we found was that the DNA itself plays a crucial role in telling the cell where to apply these methylation marks.”
This was a revelation. In Arabidopsis, a group of proteins called the CLASSYs had long been known to help guide the methylation machinery to different locations in the genome. But until now, scientists didn’t understand exactly how one of these proteins, CLASSY3, chose its targets.
The answer came with the discovery of a special class of proteins called “RIMs,” which work together with CLASSY3 to direct DNA methylation to specific regions of the genome. The RIMs, part of a larger family of transcription factors called REMs (Reproductive Meristem proteins), act as guides, docking onto stretches of DNA and instructing the methylation machinery where to land. When the researchers disrupted these DNA sequences, the entire methylation process failed.
For the first time, the team had uncovered a genetic sequence that could directly regulate DNA methylation—a discovery that promises to change the way we think about epigenetic regulation.
The Genetic Blueprint for Epigenetic Changes
“All previous work pointed to pre-existing epigenetic modifications as the starting place for targeting methylation, which didn’t explain how novel methylation patterns could arise,” explains Julie Law, Ph.D., the senior author of the study. “Now we know that the DNA itself can instruct new methylation patterns, too.”
This breakthrough challenges the traditional view of epigenetics as a system that simply “remembers” and “reinforces” existing genetic instructions. Instead, it paints a more dynamic picture, where the genetic code itself can actively shape epigenetic changes in response to developmental needs or environmental cues.
The discovery could have far-reaching implications. If scientists can learn how to control these newly identified sequences and the proteins that interact with them, it may become possible to precisely engineer DNA methylation patterns. This could lead to a host of applications in agriculture and medicine, where specific epigenetic modifications could be used to enhance plant growth or repair damaged tissues in humans.
Unlocking the Potential of Epigenetic Engineering
The implications of this discovery are profound. By using specific genetic sequences to direct DNA methylation, researchers could begin to engineer new patterns of methylation in plant and animal cells with unprecedented precision. This could help correct epigenetic defects that cause diseases like cancer, or even improve cellular functions in plants to enhance crop yields or resistance to stress.
“As we understand more about how DNA methylation patterns are created and maintained, we can begin to think about using this knowledge to improve cellular fitness,” says Law. “We could eventually use these insights to correct defective methylation patterns or to engineer new patterns that might improve health and development.”
For example, in plants, understanding and manipulating DNA methylation could allow scientists to create crops that are better adapted to changing climates or more resistant to pests. In humans, precise control over methylation could offer new ways to treat diseases that involve epigenetic malfunctions, such as certain cancers or genetic disorders.
This discovery also opens up new avenues for investigating how epigenetic changes influence development and regeneration. If DNA sequences can instruct methylation changes during reproduction or in response to environmental signals, it could lead to new insights into how organisms grow, adapt, and recover from injury.
Why This Research Matters
At its core, this study is about understanding how life’s most fundamental instructions are written, rewritten, and regulated. While genetic mutations often steal the spotlight, epigenetic modifications—how genes are turned on and off—are just as crucial in determining cellular function, identity, and behavior.
By uncovering a new mode of DNA methylation regulation, researchers have not only answered a key question in epigenetics, but they’ve also opened the door to a world of possibilities. The ability to precisely manipulate methylation patterns could revolutionize the way we approach everything from crop production to disease treatment.
This work is a step closer to harnessing the power of epigenetics to improve health, agriculture, and even our understanding of life itself. With this newfound knowledge, scientists are now equipped to explore and manipulate the very mechanisms that control our genes in ways that were once thought impossible. In the world of biology, this is a breakthrough that will echo through the future of both research and practical applications for years to come.
More information: Guanghui Xu et al, Transcription factors instruct DNA methylation patterns in plant reproductive tissues, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01808-5