For families whose children are diagnosed with brain tumors, life changes in an instant. Suddenly, ordinary concerns like school or playdates are overshadowed by medical terms, MRI scans, and urgent questions about survival. Among the most common of these childhood brain tumors is pilocytic astrocytoma (PA), which accounts for roughly 15% of all pediatric brain tumors.
On the surface, pilocytic astrocytomas are often considered “low-grade” tumors. They rarely spread to other parts of the brain or body, and with surgical removal, many children go on to live long lives. Yet these tumors are far from harmless. Because they grow within the brain—a delicate organ still developing in childhood—their presence can disrupt thinking, learning, movement, and behavior. Even when not directly life-threatening, the unchecked growth of PA cells can leave lasting scars on a child’s health and quality of life.
For years, treatment has focused on a straightforward goal: cut the tumor out. But what if the tumor is in a place too dangerous to reach with surgery? What if removing it risks damaging vital brain functions? These are the painful dilemmas families and doctors face. And until now, the options for therapy have been frustratingly limited.
Beyond the Tumor Cells: A Bigger Picture Emerges
Traditionally, cancer research has zeroed in on the tumor cells themselves. The thinking was simple—if you could stop these cells from multiplying, you could stop the disease. But recent discoveries are painting a far more complex picture. Tumors do not grow in isolation. They live within the brain’s intricate neighborhood, surrounded by neurons, support cells, and a constant flurry of chemical signals.
This realization has shifted how scientists think about pilocytic astrocytomas. Instead of viewing the tumor as a rogue, independent entity, researchers are beginning to see it as an opportunist—one that hijacks the brain’s normal communication systems to fuel its own survival and growth.
One of the most intriguing suspects in this process is glutamate, a chemical messenger that nerve cells use to communicate. Glutamate is essential for learning, memory, and the everyday function of the brain. But in cancer biology, glutamate has long been a puzzle. Across the body, it seems to encourage the growth of tumors—but exactly how it does this has remained elusive.
Cracking the Glutamate Code
A team of researchers at Washington University School of Medicine in St. Louis recently took a closer look at glutamate’s role in pediatric brain tumors. Their findings, published in Neuron, have opened up a new way of thinking about how these cancers grow—and more importantly, how they might be stopped.
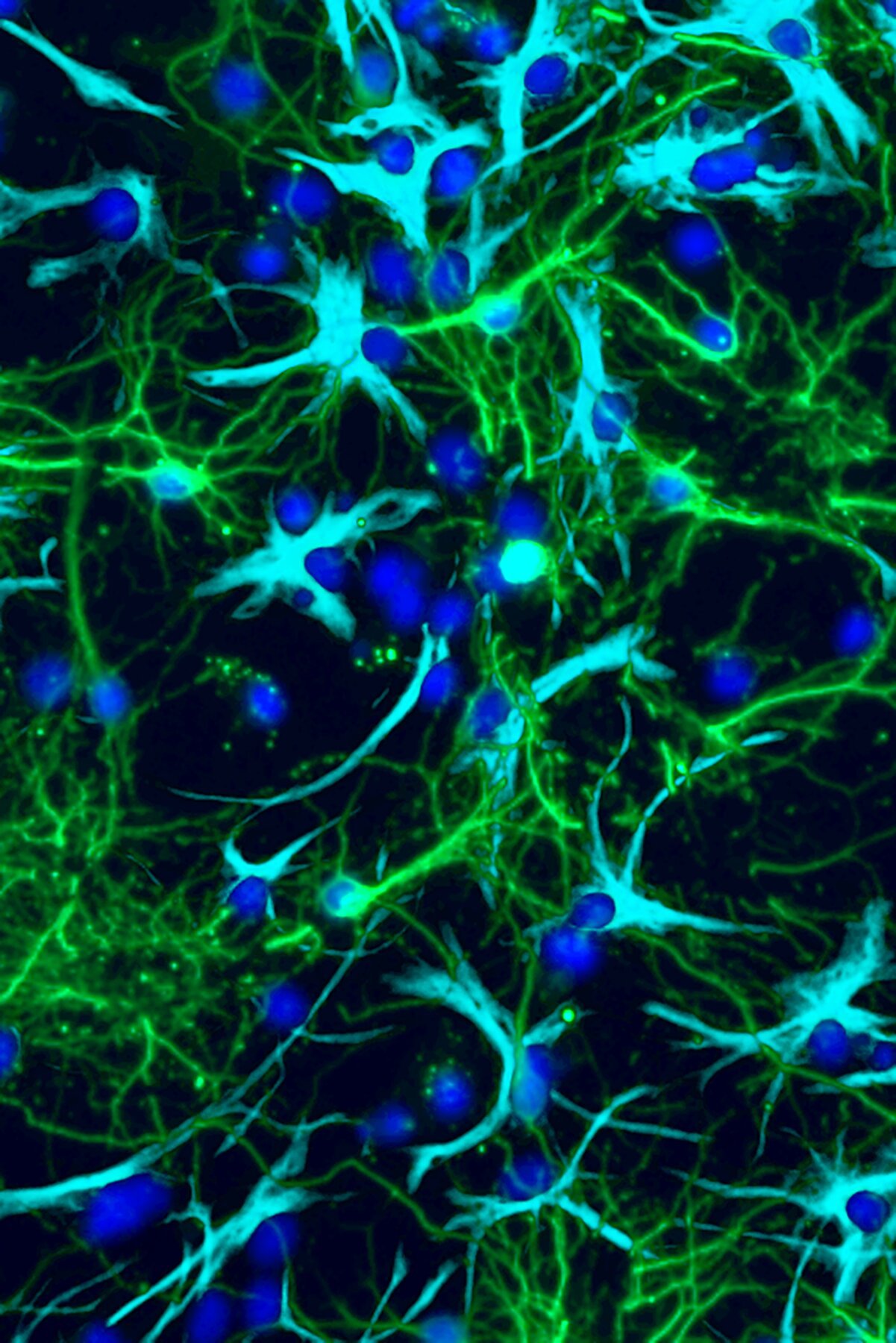
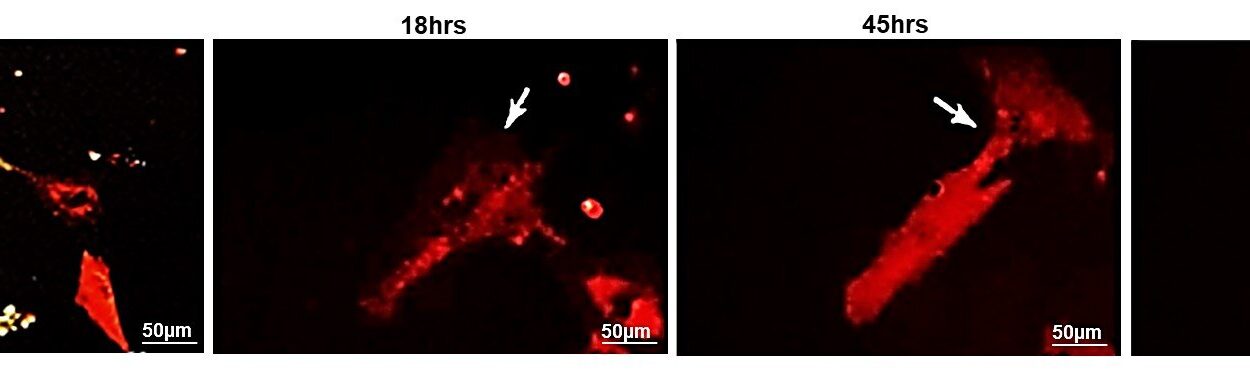
Using tumor cells isolated from patients with pilocytic astrocytoma, the researchers found something remarkable: PA cells were reprogramming glutamate receptors. Normally, these receptors sit on the surface of nerve cells, where they detect glutamate and help pass along electrical signals. But in PA cells, the receptors were no longer behaving like normal electrical switches. Instead, they had been rewired to send signals that told the tumor cells to grow and divide.
In essence, the tumor cells had stolen a page from the brain’s playbook. Instead of responding to glutamate in the usual way, they used it as fuel for uncontrolled growth. This “hijacking” mechanism revealed a direct link between the brain’s own communication system and the cancer’s relentless drive to expand.
A Familiar Drug with New Potential
Once the researchers uncovered this mechanism, the next question was obvious: could they block it? The answer, it turns out, may already be sitting in medicine cabinets around the world.
The team tested drugs known to interfere with glutamate receptors, including memantine, which is already approved to treat dementia and Alzheimer’s disease. In mice implanted with human PA cells, these drugs significantly slowed tumor growth. By blocking the abnormal signals, they cut off one of the tumor’s main growth pathways.
For senior author David Gutmann, MD, Ph.D., this finding is especially hopeful. “With these kinds of pediatric brain tumors, we just don’t have that many tools in our toolbox for treating patients,” he explained. “The potential to repurpose drugs that are already in use for other neurological disorders means we may have another trick up our sleeves for treating patients.”
The beauty of this approach lies in its practicality. Developing entirely new cancer drugs often takes decades, along with enormous costs and risks. But repurposing an existing, widely studied drug like memantine shortens that timeline considerably. If proven safe and effective for children with PAs, it could offer a much-needed treatment option within a realistic timeframe.
A New Way of Seeing Tumors
The research, led by first author Corina Anastasaki, Ph.D., did more than reveal a potential drug target. It also uncovered a fundamental insight into how tumors operate. By showing that glutamate receptors in PA cells couple with growth receptors in unusual ways, the team identified a novel mechanism for cancer progression—one that combines electrical communication and cellular growth signals in an unexpected partnership.
“This novel mechanism for tumor growth combines two normal but unconnected brain processes—growth and electrical signaling—in an aberrant way,” Anastasaki explained. This discovery not only reframes how scientists view pilocytic astrocytomas but also raises questions about whether similar processes are at work in other types of cancers.
If glutamate and other neurotransmitters can be co-opted by tumors, researchers may need to rethink cancer biology more broadly. It suggests that the brain’s unique environment—designed for communication and adaptability—can be exploited by malignant cells. This perspective could lead to new strategies for preventing and treating brain cancers in children and adults alike.
What Comes Next
While the results are promising, they are just the beginning. Testing drugs like memantine in children requires carefully designed clinical trials to determine the right dose, safety, and effectiveness. Children’s brains are still developing, and treatments must strike a delicate balance: powerful enough to curb tumor growth, but gentle enough to avoid disrupting normal brain development.
Nevertheless, the findings offer a roadmap. By understanding how tumors exploit the brain’s own chemistry, researchers can design treatments that target these vulnerabilities. Instead of blasting tumors with broad and often damaging therapies, the future may lie in precision approaches that neutralize the very signals tumors depend on.
Hope for Families and the Future
For parents and children facing the uncertainty of a brain tumor diagnosis, science like this offers more than technical progress—it offers hope. Each discovery brings us closer to treatments that are not only more effective but also less harmful, preserving the child’s ability to learn, grow, and live fully.
This research reminds us that cancer is not an invincible enemy. It is clever, adaptive, and ruthless, but it can be understood. And once understood, it can be challenged. By turning the brain’s own chemistry against the tumors, researchers are showing that the very mechanisms cancer exploits may also become its undoing.
As science moves forward, pilocytic astrocytomas may one day be more than manageable—they may be curable. And with every step, we come closer to giving children not just years of survival, but lifetimes of possibility.
More information: Aberrant coupling of glutamate and tyrosine kinase receptors enables neuronal control of brain tumor growth, Neuron (2025). doi.org/10.1016/j.neuron.2025.08.005