Schizophrenia is one of the most complex and misunderstood disorders in psychiatry. For centuries, it has puzzled physicians, mystified families, and challenged scientists. Those who live with schizophrenia often experience a disorienting break from reality — hearing voices that others cannot, holding beliefs that defy logic, or struggling to organize thoughts that once flowed easily. Yet, behind these vivid symptoms lies something far deeper than delusion or confusion. It is a condition that affects the very structure and chemistry of the brain.
Although medical science has made immense progress in understanding mental illness, schizophrenia remains a mystery. Its causes are not confined to a single gene, chemical, or life event. Instead, it seems to emerge from a complex interplay of biology, environment, and neurochemistry. For decades, researchers have tried to uncover what goes wrong inside the brain to produce such profound disturbances in thought, emotion, and perception. And now, new evidence suggests that the answer may lie in two surprising and essential components of brain health — iron and myelin.
The Brain’s Delicate Balance
Iron and myelin are not substances we usually associate with mental illness, yet both play fundamental roles in how the brain works. Iron is vital for producing neurotransmitters — the chemical messengers that allow neurons to communicate. It also helps in energy metabolism and in the creation of myelin, the fatty sheath that wraps around nerve fibers like insulation around electrical wires.
Myelin is crucial for fast, efficient signal transmission. When myelin is healthy, electrical impulses travel smoothly across neurons, allowing us to think, move, and react with incredible speed. But when myelin is damaged or deficient, communication within the brain slows down or becomes chaotic. In neurological disorders such as multiple sclerosis, myelin damage causes movement and sensory impairments. In schizophrenia, scientists have long suspected that subtle disruptions in myelin may underlie the fragmented thinking and perception that characterize the disorder.
However, the relationship between iron and myelin is intricate. Too little iron impairs brain function, but too much can cause oxidative stress — a damaging process where free radicals attack brain cells. This delicate balance makes studying iron’s role in mental illness particularly challenging.
Searching for Clues in the Living Brain
Until recently, most studies examining schizophrenia’s biological roots relied on postmortem brain tissue, offering only a static view of a dynamic disease. But thanks to modern brain imaging technologies, scientists can now peer into the living brain with unprecedented precision.
Researchers at King’s College London, Hammersmith Hospital, and Imperial College London embarked on a groundbreaking study to investigate whether abnormal levels of iron and myelin might contribute to schizophrenia. Led by Dr. Luke J. Vano, the team used a combination of advanced imaging techniques — including iron-sensitive and myelin-sensitive MRI — to map these substances in the brains of living patients.
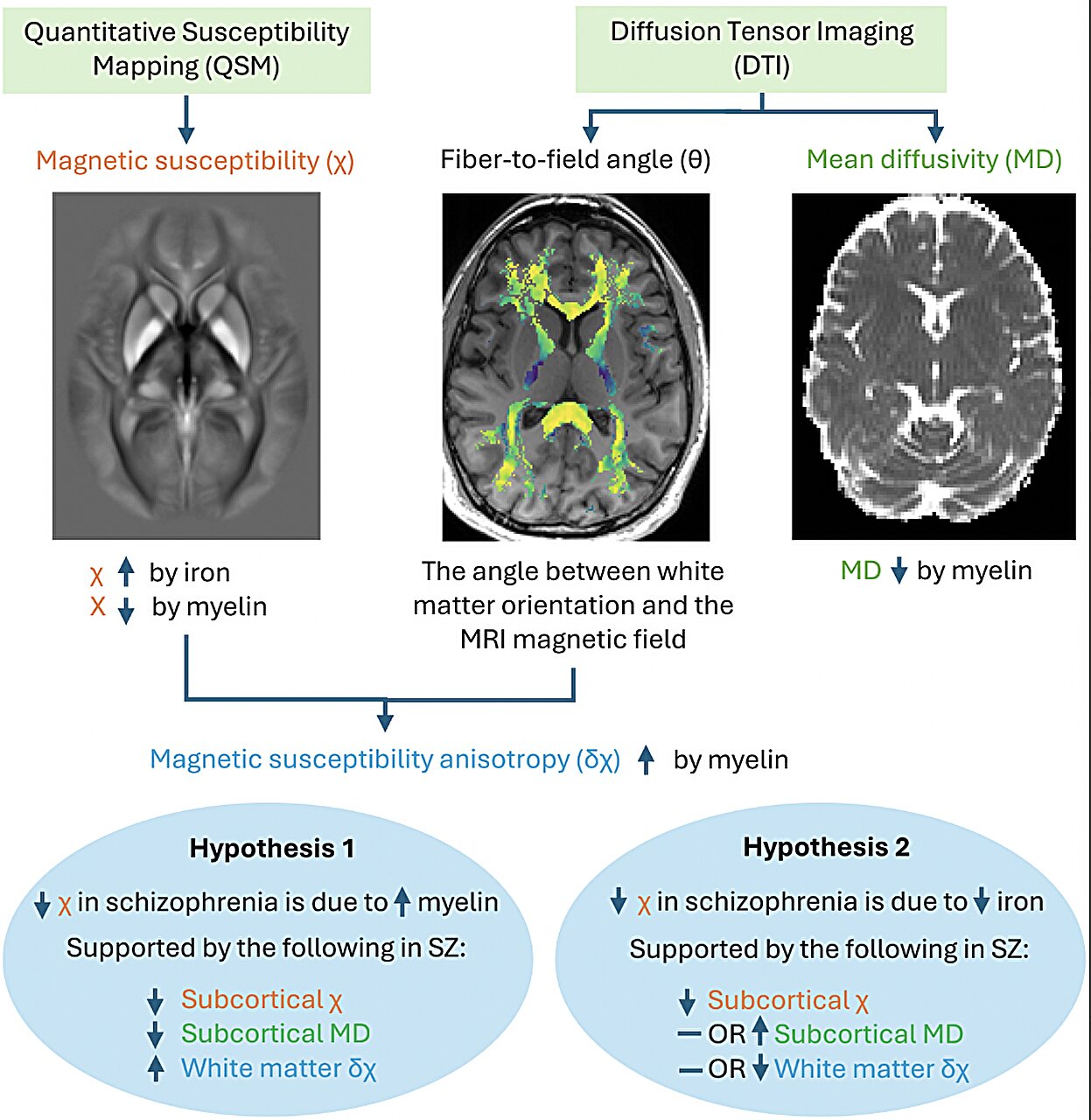
The study involved 85 individuals diagnosed with schizophrenia and 86 healthy control subjects. Using a technique called quantitative susceptibility mapping (QSM), researchers measured how magnetic properties of brain tissue changed with varying levels of iron and myelin. They also used diffusion MRI to examine how water moved through brain tissue — a measure influenced by the integrity of myelin.
The Signature of a Disorder Written in Iron and Myelin
What they discovered was striking. In several deep brain regions — the caudate, putamen, and globus pallidus — people with schizophrenia showed distinct abnormalities. These areas, part of a network involved in motivation, decision-making, and movement, displayed lower magnetic susceptibility and lower myelin-related measures. In simpler terms, both iron and myelin levels appeared reduced.
These results, published in Molecular Psychiatry, align with some earlier studies but help resolve years of conflicting evidence. Previous research had alternately reported increases or decreases in brain iron levels among patients, leaving scientists uncertain about what was really happening. Dr. Vano’s work suggests that part of this confusion may stem from myelin’s influence on iron-sensitive signals — in essence, changes in myelin can mask or mimic changes in iron.
The team also connected these findings to a specific type of brain cell: oligodendrocytes. These cells are the body’s myelin factories, responsible for wrapping neurons in protective sheaths. Oligodendrocytes depend on iron to synthesize myelin effectively. If they malfunction or lack sufficient iron, the result could be disrupted neural communication — the kind seen in schizophrenia.
The Oligodendrocyte Connection
This link between oligodendrocyte dysfunction, iron deficiency, and schizophrenia is more than a technical discovery. It offers a biological explanation for symptoms that, for too long, were dismissed as purely psychological. Disorganized speech, fragmented thinking, and emotional flatness may not simply be the result of “broken thoughts,” but of broken connections — neurons misfiring because their communication lines are poorly insulated.
It also reframes schizophrenia not as a single disease, but as a network disorder. When myelin falters, entire brain circuits can become unstable, distorting how a person perceives reality or interprets social cues. Imagine trying to watch a movie on a screen filled with static — the story becomes jumbled, the characters unrecognizable. For people with schizophrenia, that interference is constant, and it colors every experience of the world.
Toward a New Era of Diagnosis and Treatment
The implications of Dr. Vano’s findings are profound. If iron and myelin abnormalities can be detected consistently in schizophrenia, they could serve as biomarkers — measurable indicators of disease. Biomarkers could revolutionize psychiatry by allowing earlier diagnosis, better tracking of treatment responses, and more personalized therapies.
Current treatments for schizophrenia primarily target dopamine — a neurotransmitter implicated in hallucinations and delusions. While these medications can relieve some symptoms, they often leave cognitive and emotional impairments untouched. By contrast, understanding the biological foundation of myelin and iron dysfunction opens the door to entirely new therapeutic strategies.
In the future, treatments might focus on restoring healthy myelination or balancing brain iron levels. Myelin repair therapies, already in development for multiple sclerosis, could potentially be adapted for psychiatric use. Nutritional or pharmacological interventions that regulate iron metabolism may also play a role, though any such approach would need to avoid tipping the balance toward excess, which could damage the brain.
Beyond Schizophrenia: The Wider Implications
The study’s next steps will examine whether these biomarkers appear in related conditions, such as bipolar disorder, or in individuals at high risk for developing schizophrenia. If so, these findings could help identify who might benefit from early intervention — long before the first psychotic episode occurs.
More broadly, the research highlights a growing trend in neuroscience: understanding mental illness as a biological, not purely psychological, phenomenon. Just as cardiologists study the electrical rhythms of the heart, psychiatrists and neuroscientists are beginning to map the electrical and chemical rhythms of the brain.
This approach does not reduce human emotion or thought to mere chemistry; rather, it reveals how delicately chemistry supports the richness of our inner lives. The brain, after all, is both a physical organ and the seat of consciousness. To study its biology is to study the foundations of what makes us human.
The Human Side of the Science
For those living with schizophrenia, scientific advances like these bring something invaluable — hope. Schizophrenia often begins in young adulthood, striking people in the prime of their lives. It can shatter ambitions, isolate individuals from their loved ones, and make the ordinary seem impossible. Yet, research like Dr. Vano’s shows that the story of schizophrenia is not one of hopelessness, but of discovery and progress.
Every new insight into its biology brings us closer to treatments that address the disorder’s root causes rather than just its symptoms. It also helps reduce stigma by reinforcing the fact that schizophrenia is not a weakness of character or a failure of will — it is a medical condition, rooted in the brain’s complex machinery.
When families and patients understand this, compassion replaces blame, and science becomes a bridge between suffering and understanding.
The Future of Schizophrenia Research
As technology advances, scientists can explore the brain with greater detail than ever before. High-resolution imaging, genetic sequencing, and computational modeling are revealing a level of complexity that challenges old assumptions. The emerging picture of schizophrenia is not one of chaos, but of imbalance — subtle, systemic shifts in the brain’s wiring and chemistry that cascade into altered perception and thought.
Future studies will likely combine imaging, genetics, and molecular biology to map these changes from multiple perspectives. If iron and myelin prove to be consistent markers, they could eventually guide new diagnostic tools — perhaps even allowing clinicians to detect vulnerability to schizophrenia before symptoms appear.
Such progress could transform the way psychiatry is practiced. Instead of waiting for the mind to fracture, medicine could move toward prevention, supporting brain health from the start.
The Harmony Beneath the Disorder
In the end, the search for schizophrenia’s biological roots is part of a larger human quest — to understand how the brain, a fragile organ of flesh and chemistry, gives rise to thought, emotion, and self-awareness. The interplay between iron and myelin may seem like a technical detail, but it speaks to a deeper truth: even the most mysterious aspects of the mind have a material foundation.
Through studies like this, we are beginning to see that mental illness does not separate us from our biology — it reveals it. The same iron that flows through our blood, the same fatty myelin that enables us to speak and dream, are also the elements that, when disrupted, can alter reality itself.
Schizophrenia, then, is not just a story of disorder, but of imbalance — a reminder of how exquisitely delicate the harmony of the human brain truly is. And with each discovery, we move one step closer to restoring that harmony, giving millions the chance not just to survive, but to reconnect with the beautiful rhythm of their own minds.
More information: Luke J. Vano et al, The role of low subcortical iron, white matter myelin, and oligodendrocytes in schizophrenia: a quantitative susceptibility mapping and diffusion tensor imaging study, Molecular Psychiatry (2025). DOI: 10.1038/s41380-025-03195-7.