The human brain is often described as the most complex structure in the known universe. For centuries, scientists have sought to understand how billions of neurons, each with thousands of connections, work together to create thought, memory, and consciousness. Traditionally, communication between neurons was thought to occur almost exclusively at synapses—the specialized junctions where chemical and electrical signals leap across tiny gaps. Yet, science is never static. With every advance in technology, we discover that the brain is far more intricate than once imagined.
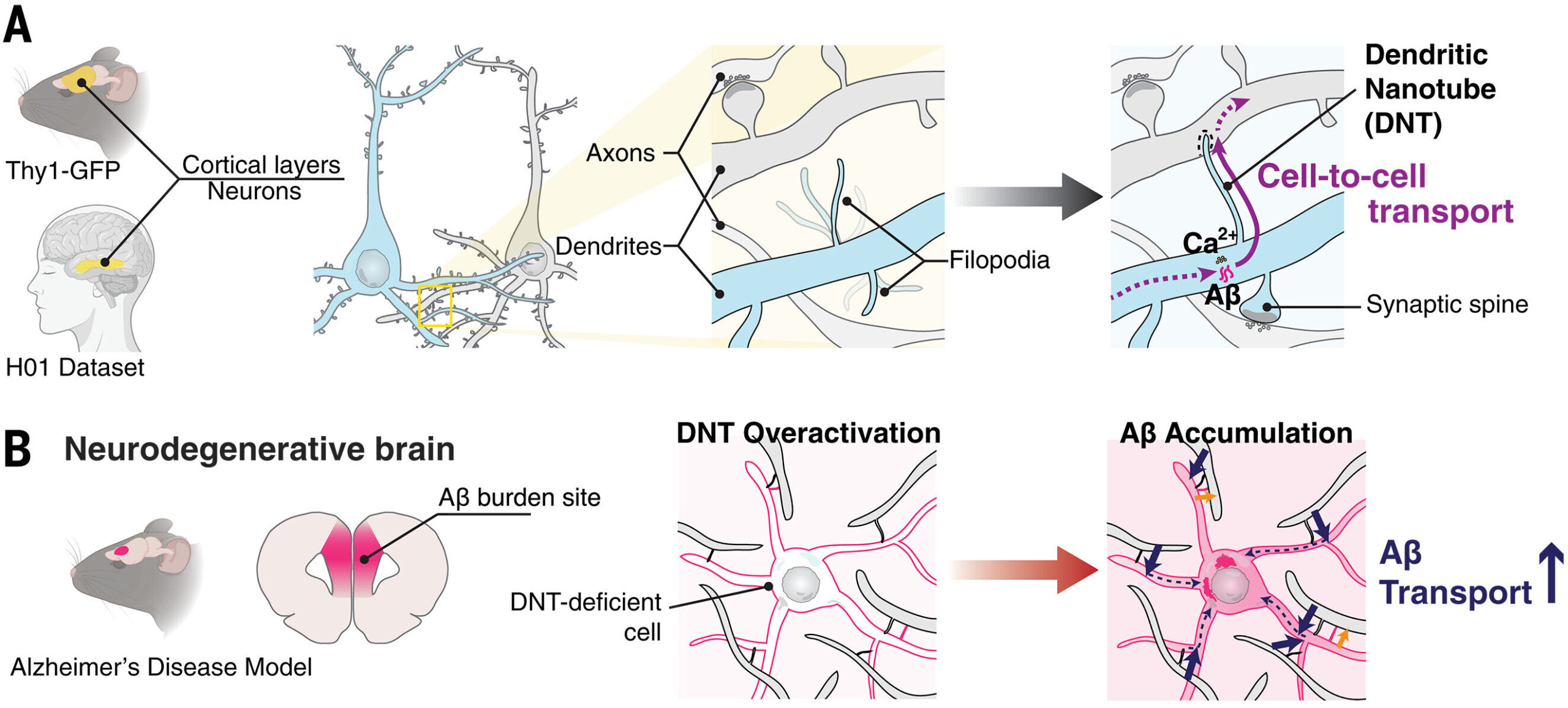
A recent study has revealed something astonishing: neurons are not just whispering to each other across synapses. They may also be using tiny physical bridges called dendritic nanotubes (DNTs) to share materials directly between their branches. This discovery doesn’t just add another chapter to the story of brain communication—it could also help us unravel one of the most devastating mysteries in medicine: Alzheimer’s disease.
Beyond Synapses: A World of Nanotubes
In recent years, researchers have learned that cells sometimes use delicate, tube-like structures to connect directly to each other. These are known as tunneling nanotubes (TNTs), and they’ve been documented in a range of cell types. TNTs act like miniature highways, transferring materials, including proteins, organelles, and even electrical signals, between cells.
But until recently, scientists weren’t sure whether such structures existed in the mature brain, where neurons are known for their sophisticated but highly specific synaptic connections. The idea that neurons could also be linked by nanotube-like bridges seemed both radical and intriguing.
Now, using state-of-the-art imaging techniques such as superresolution microscopy (dSRRF) and electron microscopy, researchers have uncovered a new variety of nanotube: the dendritic nanotube, or DNT. Unlike TNTs, which are typically open-ended tunnels, DNTs are closed at the ends, but they still allow small molecules and ions to move from one neuron to another. These bridges appear to form directly between dendrites—the tree-like branches that extend from neurons and receive signals.
The finding reframes how we think about neuronal networks. It suggests that alongside synapses, neurons might also rely on a subtler, less obvious system of connections that allow them to exchange materials in ways we are only beginning to grasp.
What Makes DNTs Different?
Distinguishing DNTs from other structures in the brain was no simple task. Dendrites are crowded with spines, filaments, and synaptic contacts, making it difficult to identify something new. To tackle this, the researchers employed machine learning algorithms trained to analyze thousands of images of neuronal structures. These tools confirmed that DNTs have unique shapes and internal architecture that set them apart from synapses and other known dendritic extensions.
The DNTs were also shown to be actin-rich, meaning their structure depends heavily on the same protein filaments that shape cells and drive movement. Observations of cultured neurons revealed that DNTs are not static—they can form and dissolve dynamically, suggesting they might respond to the brain’s changing activity or environment.
Perhaps most fascinatingly, DNTs appear to play a role in transporting calcium ions and small molecules between neurons. Calcium signaling is central to brain function, involved in processes such as learning, memory, and synaptic plasticity. If DNTs are participating in this kind of exchange, they may represent a previously hidden dimension of neural communication.
A Link to Alzheimer’s Disease
The discovery of DNTs would already be extraordinary on its own, but what makes this research especially powerful is its link to Alzheimer’s disease. Alzheimer’s is marked by the accumulation of amyloid-beta (Aβ) peptides, which clump together into plaques that damage neurons and disrupt memory. For decades, scientists have tried to understand how amyloid-beta spreads through the brain, almost like an infection that slowly engulfs neural circuits.
In this new study, the researchers injected amyloid-beta into neurons within brain slices from mice. They observed something remarkable: DNTs allowed the toxic peptides to pass from one neuron’s dendrites into the branches of nearby neurons. When the team chemically inhibited DNT formation, the spread of amyloid-beta sharply decreased.
This finding suggests that DNTs may act as conduits for disease, shuttling harmful proteins from cell to cell. If confirmed, this could fundamentally change our understanding of how Alzheimer’s progresses at the microscopic level.
Early Warnings in the Brain
The researchers went further by building computational models of how DNT networks behave during disease. Their simulations revealed that the density of DNTs increases before amyloid plaques form in the brains of Alzheimer’s model mice. In other words, these nanotube bridges may serve as an early sign of pathology, showing changes in network behavior even before the disease’s classic hallmarks are visible.
This raises exciting possibilities. If DNT activity is linked to early Alzheimer’s, therapies aimed at modifying or blocking their formation might slow the disease before irreversible damage occurs. At the very least, monitoring DNT density could provide a new biomarker for detecting Alzheimer’s in its earliest stages—when intervention is most likely to succeed.
A Double-Edged Sword
Still, it is important to remember that not all aspects of DNTs are necessarily harmful. The brain is resourceful, and new structures rarely evolve without purpose. It’s possible that under normal conditions, DNTs help neurons balance calcium, share signaling molecules, or coordinate activity in ways that enhance brain function.
In this view, Alzheimer’s may hijack a beneficial system, turning it into a pathway for toxicity. Such dual roles are common in biology: processes that protect us in one context can harm us in another. Understanding when DNTs are helpful and when they become harmful is the next big challenge.
The Tools of Discovery
What made this breakthrough possible were advances in imaging and computational analysis. Superresolution microscopy allows scientists to visualize structures far smaller than the diffraction limit of light, while electron microscopy provides detailed ultrastructural snapshots. Together, they revealed the delicate bridges weaving between dendrites—features invisible to traditional methods.
Equally critical was the use of machine learning. By training algorithms to recognize subtle patterns that the human eye might miss, researchers could confidently distinguish DNTs from other dendritic features. This marriage of biology and artificial intelligence is becoming a cornerstone of modern neuroscience, opening doors to discoveries that would once have seemed impossible.
Looking Ahead
Much remains unknown about dendritic nanotubes. Do they appear throughout the brain, or only in certain regions? What molecules do they normally transport? Do they influence learning and memory? How do they form and dissolve in response to activity? And most urgently: how can we harness this knowledge to fight Alzheimer’s disease and related dementias?
Future research will likely focus on answering these questions, using both animal models and human brain tissue. If DNTs prove to be major players in neurodegeneration, targeting them could become a revolutionary therapeutic strategy. On the other hand, if they also support healthy brain function, therapies will need to be carefully designed to preserve their beneficial roles while blocking harmful ones.
A New Frontier in Understanding the Brain
The discovery of dendritic nanotubes is a reminder of just how much we still have to learn about the brain. For all our progress, the brain’s inner workings remain as mysterious and awe-inspiring as the cosmos. Each discovery peels back one layer of the puzzle, revealing new structures, new mechanisms, and new ways in which life organizes itself at the smallest scales.
For those touched by Alzheimer’s disease—patients, families, caregivers—this discovery brings a spark of hope. It points to a fresh direction in the search for answers, offering not just another piece of the puzzle, but the possibility of changing how we approach the disease from its earliest stages.
Science often advances in small steps, but sometimes, it leaps forward with a discovery that reshapes the landscape of knowledge. Dendritic nanotubes may be one of those leaps—a hidden bridge in the brain that, once illuminated, could help us understand not only the tragedy of Alzheimer’s but also the extraordinary complexity of the organ that makes us who we are.
More information: Minhyeok Chang et al, Intercellular communication in the brain through a dendritic nanotubular network, Science (2025). DOI: 10.1126/science.adr7403