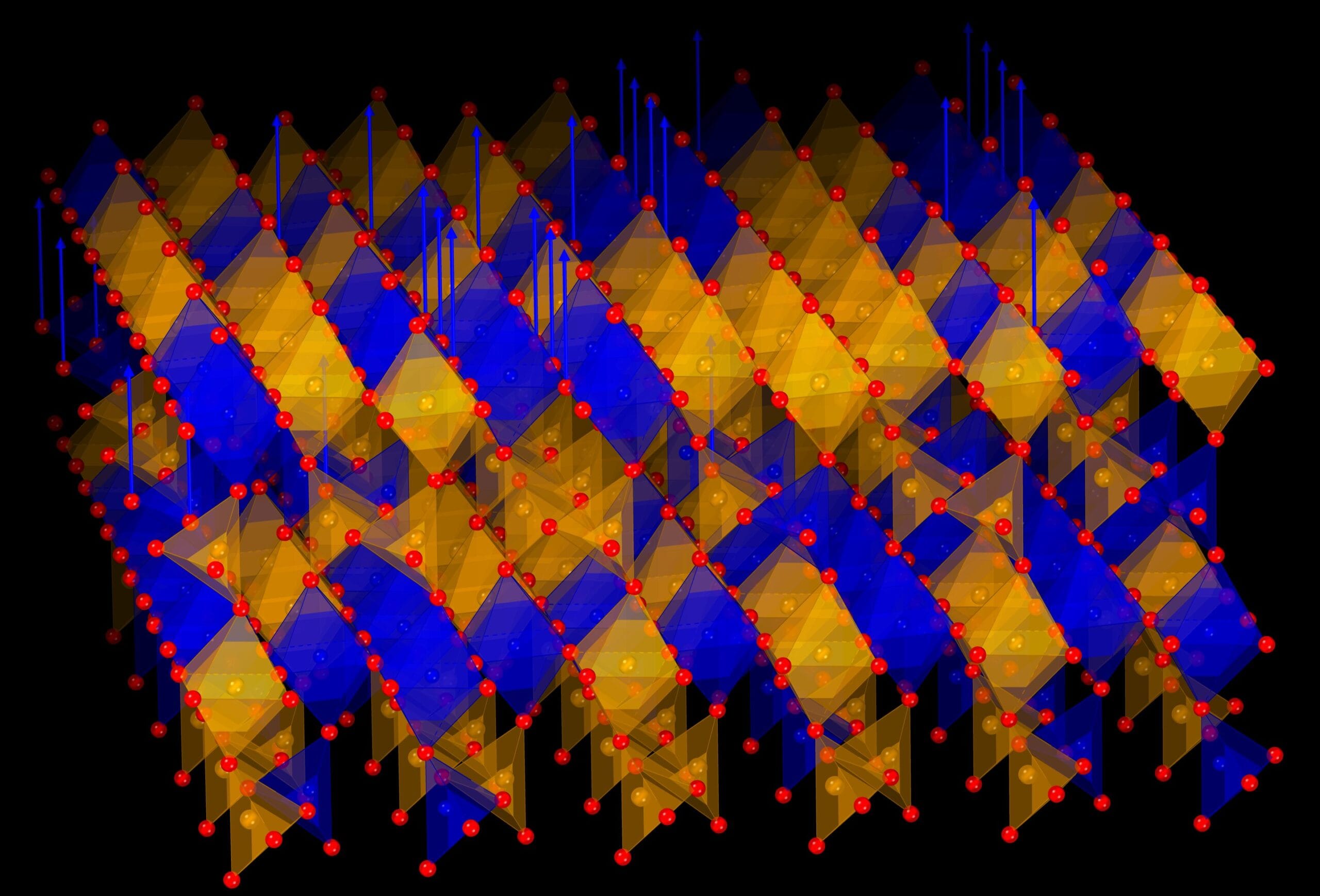
Imagine a crystal that breathes — not metaphorically, but literally. A team of researchers from Korea and Japan has unveiled a remarkable new material that can release and reabsorb oxygen over and over again, almost like lungs filling and emptying with air. Unlike fragile compounds that break down after a few cycles or require searing heat to function, this new crystal operates at relatively low temperatures and remains stable through repeated use.
The discovery, published on August 15, 2025, in Nature Communications, marks a breakthrough in material science with enormous potential for clean energy technologies.
“It is like giving the crystal lungs and it can inhale and exhale oxygen on command,” says Professor Hyoungjeen Jeen, who led the study at Pusan National University in Korea.
How It Works: Oxygen on Demand
The material is a specially engineered metal oxide composed of strontium, iron, and cobalt. When gently heated in a controlled gas environment, the crystal releases oxygen from its structure. Then, when exposed again to oxygen-rich conditions, it reabsorbs the gas and returns to its original form.
What makes this material extraordinary is its resilience. This oxygen in-and-out process, which scientists call oxygen redox cycling, can be repeated many times without the crystal falling apart. The secret lies in its unique atomic arrangement: only cobalt ions shift their chemical state during the process, while the overall framework of the crystal transforms into a new but stable structure.
“This finding is striking in two ways: only cobalt ions are reduced, and the process leads to the formation of an entirely new but stable crystal structure,” explains Prof. Jeen.
Why Oxygen Control Matters
Oxygen is not just vital for life — it is also at the heart of some of the most important clean technologies. The ability to control how materials store and release oxygen determines the efficiency of devices such as:
- Solid oxide fuel cells, which generate electricity from hydrogen with minimal emissions.
- Thermal transistors, which control heat in the same way electrical transistors control current.
- Smart windows, which automatically adjust how much heat they let in or block depending on the weather.
Until now, most materials capable of such oxygen control required extreme conditions, like temperatures above 800°C, or they degraded quickly after repeated cycles. This new crystal changes the game by operating at far milder temperatures while staying structurally sound.
Toward Smart, Adaptive Materials
The reversibility of the process is key. The researchers demonstrated that after oxygen was released, the crystal could fully return to its original state once oxygen was reintroduced — a sign of its long-term stability.
“This is a major step toward the realization of smart materials that can adjust themselves in real time,” says Professor Hiromichi Ohta, co-author from Hokkaido University in Japan.
Such adaptability hints at a future where buildings regulate their own heat flow, where energy devices run more efficiently, and where everyday technologies breathe in harmony with their environments.
A Breath of the Future
While still in the early stages of research, the implications are vast. Clean energy generation, heat management in electronics, and eco-friendly architecture could all benefit from a material that “breathes” on command.
The discovery also represents something more profound: a reminder that even crystals — seemingly lifeless, rigid objects — can exhibit behaviors that mirror life itself when probed with the right insight.
As Prof. Jeen puts it, “We are learning to teach materials how to live, at least in the way they interact with their surroundings. This crystal shows us what is possible.”
In the coming years, as scientists refine and scale this discovery, the breathing crystal may well become a cornerstone of technologies that make energy cleaner, smarter, and more sustainable.
More information: Joonhyuk Lee et al, Selective reduction in epitaxial SrFe0.5Co0.5O2.5 and its reversibility, Nature Communications (2025). DOI: 10.1038/s41467-025-62612-1. www.nature.com/articles/s41467-025-62612-1