In the modern age, where every gadget from your smartphone to your electric vehicle relies on stored energy, the humble battery has emerged as the unseen engine driving our technological lives. These unassuming little cylinders and rectangles hold within them a world of fascinating physics—an elegant dance of electrons, ions, and chemistry wrapped in modern materials and human ingenuity. But how exactly do batteries work? What allows a battery to power a flashlight for hours or drive a car for hundreds of miles? The answer lies in the invisible forces of the atomic world, where physics and chemistry intertwine to store and deliver energy on demand.
To understand the magic inside a battery, we must journey into the realms of thermodynamics, electrochemistry, and electromotive force. We’ll break open the black box of the battery and explore not just what happens, but why it happens, following the trail of energy from chemical bonds to glowing light bulbs and spinning motors.
The Heart of the Battery: A Primer in Energy
Before diving into the battery itself, we must first grasp what energy is in the physical sense. Energy, in all its various forms, is the ability to do work. In physics, “work” means any transfer of energy that results in movement against a force—lifting a weight, moving a charge, spinning a wheel.
Batteries are unique because they store energy chemically, not mechanically or thermally. This stored chemical energy is potential energy—energy waiting to be unleashed. Inside a battery, this energy is stored in the chemical bonds of the materials in its electrodes. The trick is to design a system where these materials can undergo reactions that release this energy in a controlled way—specifically, through the movement of electrons from one place to another. And therein lies the genius of the battery.
Enter Electrochemistry: Where Chemistry Meets Electricity
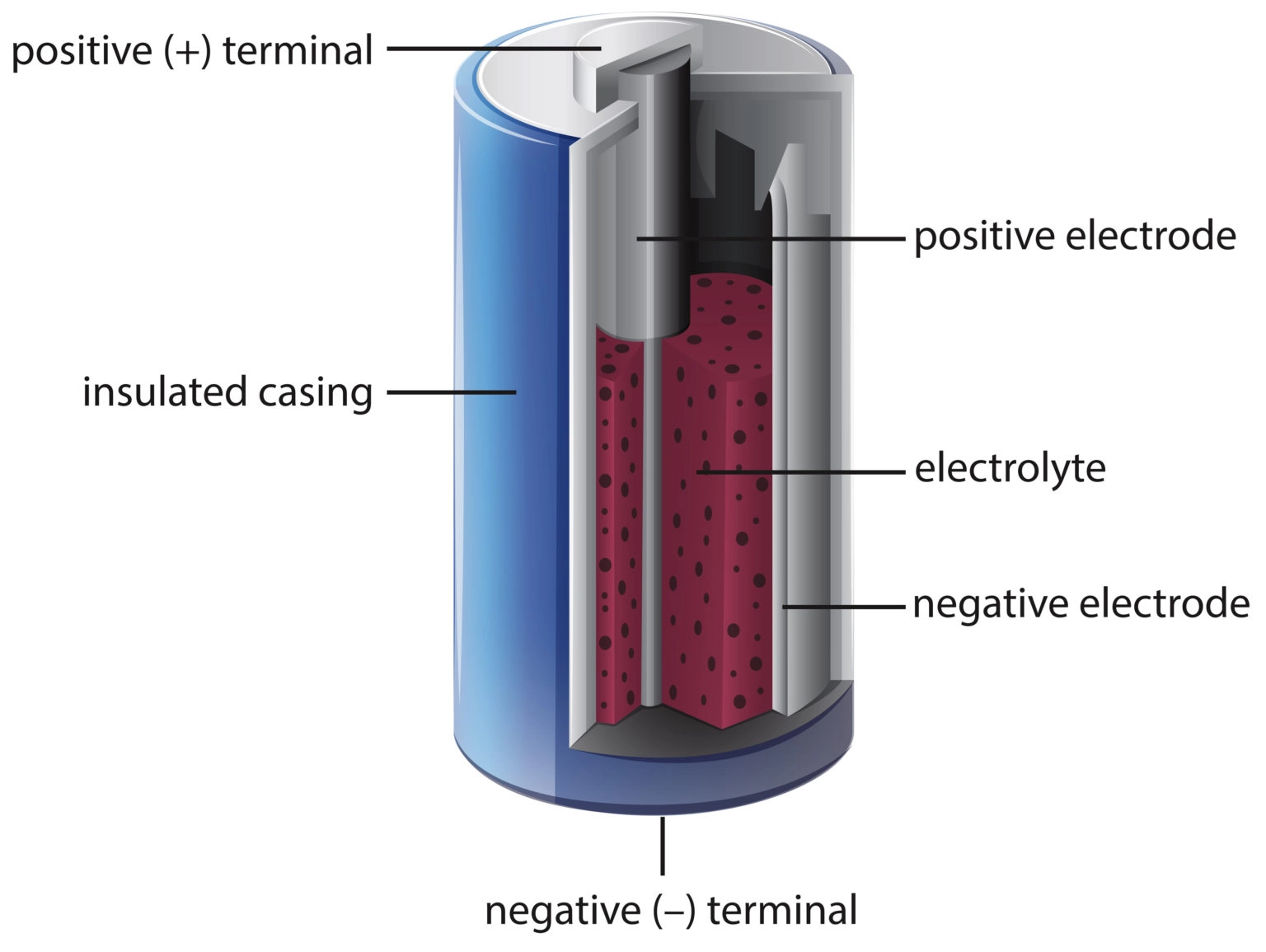
A battery is essentially an electrochemical cell, a device that converts chemical energy into electrical energy. The basic building blocks of any battery include two electrodes—called the anode and the cathode—and an electrolyte, a medium that allows ions to move between the electrodes.
At the atomic level, the process begins with redox reactions—short for reduction and oxidation. Oxidation occurs when a substance loses electrons, while reduction occurs when a substance gains electrons. In a battery, the anode is where oxidation happens: electrons are stripped away from atoms. These free electrons are then forced to travel through an external circuit to the cathode, where reduction takes place: atoms there accept the incoming electrons.
This flow of electrons through the external circuit is what we harness as electricity.
The Electric Circuit: More Than Just Wires
When we connect a battery to a circuit—say, a flashlight—the battery doesn’t just “turn on.” What happens is a beautifully orchestrated movement of charges. Electrons released at the anode flow through the circuit, doing electrical work—lighting up bulbs, powering chips—before arriving at the cathode.
But electrons don’t move in isolation. In the battery itself, there must be a way for the circuit to be completed internally. This is where the electrolyte comes in. While electrons travel through the wire, ions—charged atoms or molecules—move through the electrolyte from one electrode to the other, maintaining charge balance.
This synchronized movement of electrons externally and ions internally is what keeps the electrochemical reaction—and the battery—going.
The Components: Anode, Cathode, and Electrolyte
Let’s dig deeper into each of these components.
The anode, often made from metals like lithium or zinc, is the site where oxidation occurs. These materials are chosen because they are willing to give up electrons easily. The electrons freed from the anode travel through your device before being accepted at the cathode.
The cathode is typically made from compounds that are good electron acceptors—such as manganese dioxide or lithium cobalt oxide. These materials can incorporate the incoming electrons into their structure, completing the redox reaction.
The electrolyte, which may be a liquid, gel, or solid, must be capable of allowing ions to flow while preventing electrons from taking a shortcut through the battery, which would cause a short circuit. It’s a medium, a bridge, and a gatekeeper all in one.
Voltage: The Push Behind the Flow
Voltage is the measure of electrical potential difference between the anode and cathode. Think of it as the pressure behind the electrons. The greater the difference in the energy levels of the electrons in the two electrodes, the higher the voltage.
Each chemical reaction pair in a battery generates a specific voltage. For instance, a zinc-carbon battery typically produces about 1.5 volts per cell, while a lithium-ion cell might produce around 3.7 volts. This is why batteries are often stacked in series inside devices—to add up to a higher total voltage.
The Birth of the Battery: A Spark from the Past
The concept of the battery dates back to the late 18th century, when Italian scientist Alessandro Volta constructed the first true battery, the voltaic pile. His invention consisted of alternating layers of zinc and copper separated by pieces of cloth soaked in saltwater. It was primitive, but it worked—it produced a steady electric current. This early work laid the foundation for modern electrochemistry and the birth of portable electricity.
Volta’s pile worked because of the same principles we use today: different metals have different tendencies to give up or accept electrons. By arranging them cleverly and providing a path for both electrons and ions to move, Volta created the first controllable source of electric current.
Modern Marvels: Alkaline to Lithium-Ion
Today, batteries come in many shapes, sizes, and chemistries. Alkaline batteries, commonly used in household devices, rely on zinc and manganese dioxide. They’re reliable, cheap, and safe, but not rechargeable.
Rechargeable batteries—like nickel-metal hydride (NiMH), nickel-cadmium (NiCd), and lithium-ion (Li-ion)—operate on the same principles but use different materials that can be returned to their original state via charging.
In a rechargeable battery, the chemical reactions that produce electricity can be reversed by applying an external voltage. This forces electrons to move in the opposite direction, restoring the original chemical states of the electrodes. This cycle can be repeated hundreds or even thousands of times, depending on the battery’s design.
Lithium-ion batteries, in particular, have revolutionized portable electronics. With their high energy density, lightweight design, and long life, they power everything from phones to cars to spacecraft. The physics behind them is intricate, involving layers of nanostructured materials and precise control of ionic diffusion paths.
Energy Density and Efficiency
One of the most important metrics for batteries is energy density—how much energy a battery can store per unit mass or volume. This determines how long your phone lasts between charges or how far an electric car can go.
Physics sets limits on energy density based on the materials used and the fundamental thermodynamics of their reactions. Lithium, for example, is extremely light and has a high electrochemical potential, making it ideal for high-energy-density batteries.
Efficiency is another key concern. Not all the energy stored in a battery is recoverable. Some is lost as heat due to internal resistance. Engineers strive to design batteries with low resistance and minimal self-discharge (where the battery loses charge even when not in use).
The Charging Process: Reversing the Flow
Charging a battery is essentially running the redox reactions in reverse. An external power source pushes electrons back into the anode and pulls them from the cathode. At the same time, ions in the electrolyte move in the opposite direction, restoring the original chemical composition.
This requires precise voltage control. Too little voltage and the reaction won’t occur; too much, and you risk damaging the battery or causing unwanted side reactions. Overcharging can lead to gas buildup, overheating, and in the worst cases, fire or explosion.
Lithium-ion batteries are particularly sensitive and include built-in circuits to prevent overcharging and excessive discharging. Their charging curves follow specific profiles, with constant current followed by constant voltage, ensuring safety and longevity.
Degradation: Why Batteries Don’t Last Forever
Despite their apparent robustness, batteries degrade over time. Each charge-discharge cycle causes slight structural changes in the electrode materials. Tiny cracks form, ions get trapped, and chemical impurities accumulate. The result is a gradual loss of capacity.
Thermal effects also play a role. High temperatures accelerate chemical reactions that degrade the electrolyte and corrode the electrodes. Cold temperatures slow down ion movement, reducing performance temporarily.
Scientists are exploring materials like solid electrolytes, silicon anodes, and lithium-sulfur chemistries to combat these issues and extend battery life.
Physics in the Future: What’s Next for Batteries?
As our energy demands grow, so does the need for better batteries. Physicists and engineers are exploring new frontiers in materials science and nanotechnology to build the next generation of energy storage.
Solid-state batteries, which replace the liquid electrolyte with a solid one, promise higher energy density and improved safety. Quantum batteries—a concept still largely theoretical—envision energy storage at the level of quantum states, potentially allowing ultra-fast charging.
Flow batteries, meanwhile, separate the energy-storing chemicals from the electrodes, allowing for easier scaling in grid-level storage. These might become vital for balancing renewable energy inputs from solar and wind power.
A Universe of Tiny Engines
At its core, a battery is a self-contained universe of tiny engines—atoms undergoing transformations, pushing electrons through wires, and powering the vast ecosystem of modern life. It’s a marvel of physics and chemistry, a triumph of human understanding of the fundamental forces that govern matter and energy.
When you tap your phone screen, flip on a flashlight, or drive a silent electric vehicle, you’re witnessing the silent ballet of ions and electrons, choreographed by the principles of thermodynamics and electrostatics. You’re using the physics of stored energy—a legacy stretching back to Volta, shaped by Faraday and Maxwell, and still evolving today.
In a world that increasingly runs on portable power, the battery is no longer a luxury—it’s a necessity. And as we chase dreams of electric flight, energy-autonomous homes, and interplanetary travel, the battery remains the beating heart of those aspirations.
The physics of stored energy is far from simple. But it’s nothing short of miraculous. The next time you see a battery, remember that inside it, invisible forces are at play—silent, patient, and incredibly powerful. That is the physics of stored energy. That is how batteries work.