In the quiet corridors of TU Wien, something astonishing has occurred. A team of scientists, working with a cloud of ultracold rubidium atoms, has observed a behavior in matter that breaks the rules we’ve known for centuries. Transport, the flow of energy and mass through materials, is a concept so fundamental to the physical world that we encounter it every day—electricity flowing through wires, heat traveling through metal, water coursing through pipes. Each of these familiar flows slows down over time, hindered by resistance. But in this experiment, something extraordinary happened: resistance didn’t show up at all.
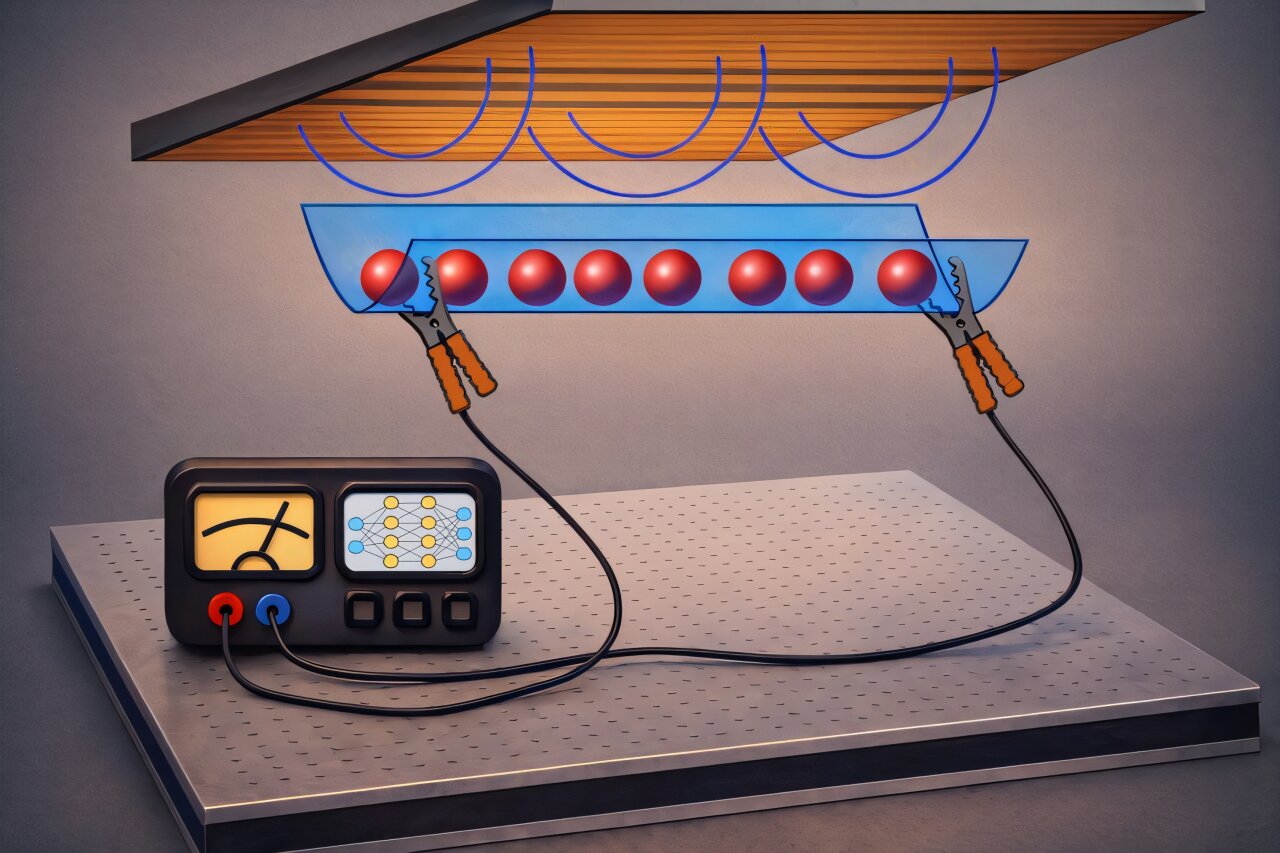
Imagine a system where particles, even after countless collisions, continue to move through a material with perfect efficiency. No friction, no resistance, just smooth, uninterrupted flow. That’s exactly what the scientists observed with their ultracold quantum gas—a system of rubidium atoms confined to a single line using magnetic and optical fields.
Published in Science, the experiment revealed a world where energy and mass flow freely, undiminished by the usual laws of resistance. This was no ordinary matter, and it opened a door to an entirely new understanding of how transport works on the quantum level.
When Transport Breaks Down
In everyday life, when you turn on a light, heat something up, or send an electric current through a wire, you’re witnessing the effects of transport. Matter, energy, or charge moves through a material, but in every case, resistance hampers that flow. Whether it’s the resistance of the wire, the friction between particles, or the energy lost to heat, transport is never perfect.
But there are two kinds of transport: ballistic and diffusive. “In principle, there are two very different types of transport phenomena,” explains Frederik Møller, one of the lead researchers on the project. “We speak of ballistic transport when particles move freely and cover twice the distance in twice the time—like a bullet traveling in a straight line.”
On the other hand, there’s diffusive transport, which is slower and more erratic. Think about heat conduction: when some hot particles collide with cooler ones, they gradually share energy until everything reaches the same temperature. This process is not linear—traveling double the distance typically takes four times as long, because each interaction loses some energy.
But in this new experiment, the rubidium atoms behaved in a way that defied both of these expectations. “By studying the atomic current, we could see that diffusion is practically completely suppressed,” says Møller. “The gas behaves like a perfect conductor; even though countless collisions occur between the atoms, quantities like mass and energy flow freely, without dissipating into the system.”
A Newton’s Cradle in the Atomic World
To understand why this happens, the team turned to a familiar analogy: Newton’s cradle. This classic desk toy, with its row of swinging metal balls, helps to illustrate how momentum is transferred from one object to another without loss. When you pull one ball back and release it, its momentum is transferred straight through the others, and the last ball swings out with the same force, as if untouched.
“The atoms in our system can only collide along a single direction,” explains Møller. “Their momenta are not scattered but simply exchanged between collision partners. Each atom’s momentum remains conserved—it can only be passed on, never lost.” Just like the Newton’s cradle, the motion in this atomic system continued without damping. The atoms passed their momentum and energy along, without any of it dissipating into the gas itself.
This discovery is revolutionary because it challenges the fundamental laws that govern how we understand energy and mass flow in everyday materials. Normally, when atoms collide, they scatter energy and momentum in all directions, causing a gradual loss of order and the eventual breakdown of transport. But in this ultracold quantum gas, the process continued smoothly, much like a perfectly synchronized series of transfers in a Newton’s cradle.
A Glimpse Into the Quantum Unknown
The experiment’s results raised a host of fascinating questions about the nature of quantum systems. “These results show why such an atomic cloud does not thermalize—why it doesn’t distribute its energy according to the usual laws of thermodynamics,” says Møller. In normal materials, energy flows are chaotic, gradually equalizing until equilibrium is reached. But in this quantum gas, the atoms didn’t distribute their energy. Instead, it remained confined, flowing along in a way that doesn’t obey the typical rules.
This behavior offers a rare, controlled look into the mechanics of transport at the quantum level. In a sense, it’s as if the atoms had learned to behave in an entirely different way, one that defies the conventional understanding of resistance and dissipation. Studying this kind of transport under such perfectly controlled conditions could provide groundbreaking insights into how resistance actually emerges—or disappears—on the quantum scale.
For years, physicists have grappled with understanding how resistance works in quantum systems, and this discovery offers new possibilities. Could this kind of perfect, lossless transport exist in other quantum systems? How might we harness this phenomenon for new technologies, from energy-efficient materials to cutting-edge quantum computing?
Why This Research Matters
This research matters because it opens a window into a world we’ve barely begun to understand: the quantum realm. In our everyday lives, resistance is a given. It’s why your phone heats up when you use it too much, why electrical wires get warm, and why friction wears down surfaces over time. But in this experiment, resistance is nearly nonexistent, and that could have profound implications for future technologies.
Imagine devices that can transport energy or mass without any loss. Imagine building materials that carry electricity without generating heat. The possibilities for energy efficiency, quantum computing, and other advanced technologies are limitless. As we push deeper into the quantum realm, discoveries like this could pave the way for the next generation of breakthroughs, ones that might change how we think about and use energy forever.
By exploring how atoms behave in such extreme conditions, we may find new principles that challenge our very understanding of matter itself. This isn’t just science fiction—it’s the future unfolding before us, and the road to that future might just begin with the fascinating, frictionless flow of rubidium atoms.
More information: Philipp Schüttelkopf et al, Characterizing transport in a quantum gas by measuring Drude weights, Science (2025). DOI: 10.1126/science.ads8327. On arXiv: DOI: 10.48550/arxiv.2406.17569