As humanity steps deeper into an age powered by electric vehicles, renewable energy, and digital technology, the world’s hunger for efficient and sustainable energy storage has never been greater. From smartphones to satellites, nearly everything that defines modern life depends on batteries — the silent engines driving our portable and renewable future. Yet, as demand accelerates, a troubling truth emerges: our reliance on lithium, the core ingredient of modern batteries, is becoming increasingly unsustainable.
Enter a quiet revolution from Japan — one that could redefine the future of energy. Researchers at Tohoku University have achieved a scientific milestone by developing a prototype rechargeable magnesium battery (RMB) that addresses many of the challenges which have long hindered magnesium-based energy storage. Their findings, published in Communications Materials, reveal a glimpse of what could be the next generation of safe, sustainable, and high-performance batteries.
The Lithium Problem
For decades, lithium-ion batteries have powered our lives. They are lightweight, energy-dense, and reliable — but they also come at a cost. Lithium is not an infinite resource; its extraction requires intensive mining, often with environmental and geopolitical consequences. As electric vehicles and renewable energy storage surge in demand, global lithium supplies are under immense pressure.
Tohoku University researcher Tetsu Ichitsubo explains the dilemma: “Lithium is a scarce resource, which makes it difficult to produce enough lithium-ion batteries to keep up with new technology and our ever-expanding population.”
In contrast, magnesium — the eighth most abundant element in the Earth’s crust — offers a sustainable alternative. Found abundantly in minerals and seawater, magnesium is inexpensive, safe to handle, and environmentally friendly. It promises a path toward large-scale, eco-conscious battery production. Yet, for all its potential, magnesium has faced one stubborn obstacle: sluggish ion movement.
The Challenge of Magnesium
For years, researchers have been intrigued by magnesium’s potential. Each magnesium atom carries two positive charges (Mg²⁺), theoretically allowing it to store more energy than a lithium atom, which carries only one. In principle, this could make magnesium batteries even more energy-dense than their lithium counterparts.
However, the reality has been less kind. Magnesium ions are heavier and more tightly bound than lithium ions, meaning they move slowly within battery materials. This sluggish ion diffusion has prevented magnesium batteries from functioning efficiently at room temperature.
Ichitsubo puts it simply: “Imagine if your device batteries could only function in extreme temperatures. It would be essentially useless for day-to-day life.”
Overcoming this limitation — achieving room-temperature magnesium battery operation — has long been the holy grail of magnesium battery research. And now, it seems, scientists may have finally cracked the code.
The Breakthrough: A New Kind of Cathode
The key to Tohoku University’s success lies in a newly designed amorphous oxide cathode with a complex but elegant formula: Mg₀.₂₇Li₀.₀₉Ti₀.₁₁Mo₀.₂₂O. This material enables a crucial process — the smooth movement of magnesium ions at room temperature.
In earlier magnesium battery designs, the ions would often become trapped or move too slowly through the cathode material, leading to poor performance and short lifespans. The new amorphous oxide, however, changes that dynamic entirely.
It uses an ion-exchange mechanism between lithium and magnesium that creates open pathways, or “diffusion channels,” through which magnesium ions can move freely. These structural pathways allow the battery to reversibly insert and extract magnesium ions, maintaining efficiency over many charge-discharge cycles.
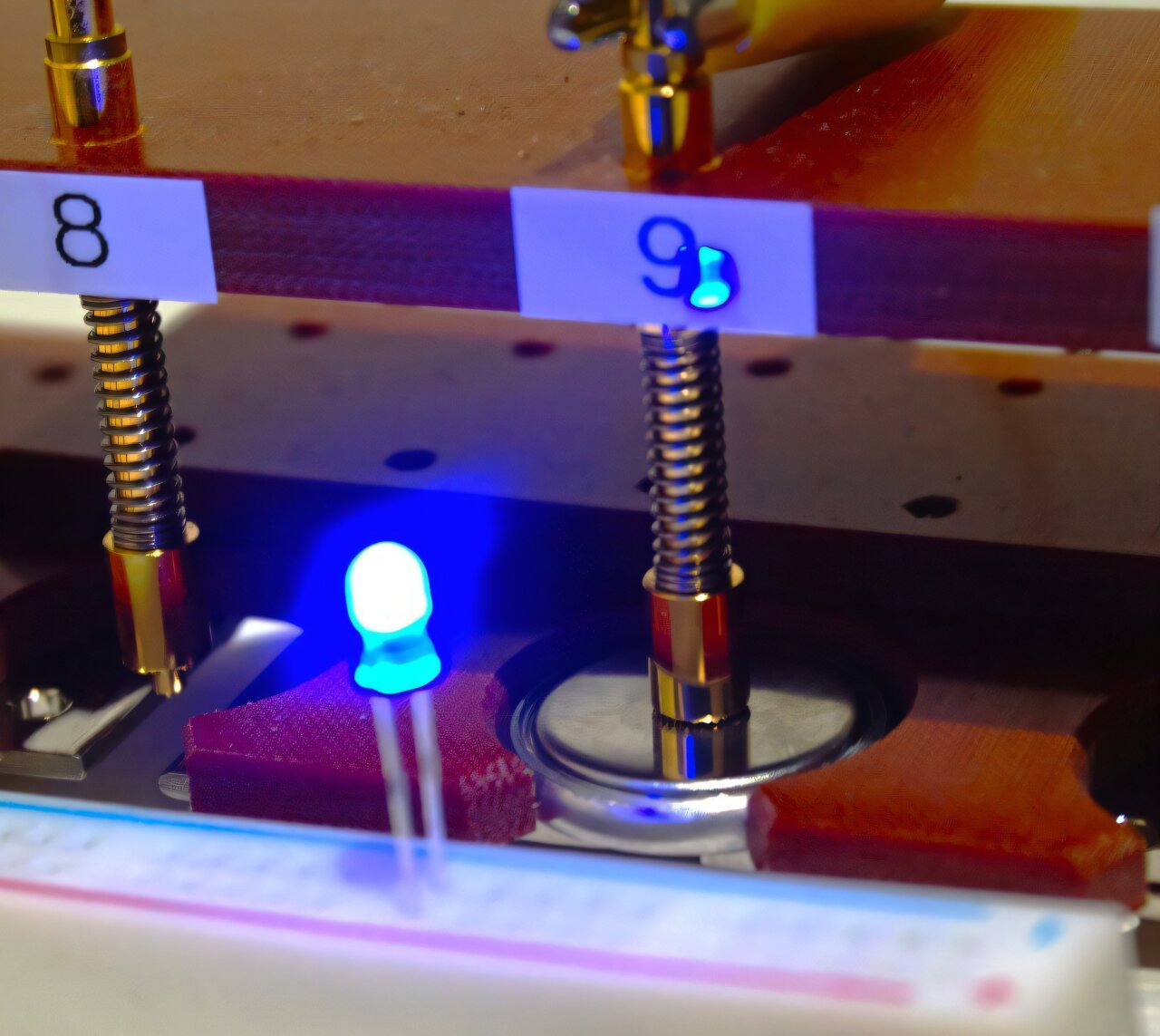
This design doesn’t just make the battery functional — it makes it practical. The researchers built a prototype full cell, tested it under realistic conditions, and found it could deliver consistent energy output even after 200 cycles.
“It was enough to continuously power a blue light-emitting diode (LED),” says Ichitsubo. “This is exciting, because previous demonstrations of RMBs showed negative discharge voltages, which means they failed to deliver usable energy.”
In other words, this prototype doesn’t just work in the lab — it works in the real world.
Verified Performance: True Magnesium Intercalation
One of the most striking aspects of this study is its rigorous validation. In past magnesium battery experiments, researchers often observed what appeared to be successful charge and discharge cycles, but deeper analysis revealed that side reactions — not true magnesium ion movement — were responsible for the results.
Tohoku University’s team took a different approach. They conducted comprehensive chemical analyses to confirm that the battery’s performance truly stemmed from magnesium intercalation, meaning magnesium ions were genuinely moving into and out of the cathode structure.
This distinction is crucial. It confirms that the observed energy capacity comes from authentic magnesium-ion behavior rather than false positives. Such confirmation elevates the research from theoretical curiosity to verified technological achievement.
The Science Behind the Success
What makes this amorphous oxide cathode so special? The answer lies in the microstructure. Traditional crystalline materials, like those used in lithium-ion batteries, have rigid, well-ordered atomic arrangements. These can be excellent for lithium but restrictive for larger ions like magnesium.
Amorphous materials, on the other hand, lack such rigid structures. Their atoms are arranged more loosely, creating “free volume” — tiny pockets of space that allow ions to move more freely. This flexibility helps overcome the slow ion diffusion that has long plagued magnesium-based systems.
The Tohoku University design also incorporates elements such as titanium and molybdenum, which contribute to the cathode’s electronic conductivity and stability. Moreover, the researchers engineered the particles at the nanoscale, further enhancing ion movement and minimizing structural degradation during repeated cycling.
Together, these design strategies form a blueprint for the next generation of sustainable batteries — ones that could rival or even surpass today’s lithium-ion technology.
Sustainable Energy for a Sustainable Future
The implications of this breakthrough reach far beyond the laboratory. A future powered by rechargeable magnesium batteries could dramatically reshape global energy systems.
Magnesium’s abundance means the raw material supply would not be limited by geography or geopolitics. It can be extracted from widely available minerals and even seawater, drastically reducing environmental impact compared to lithium mining. Furthermore, magnesium batteries are inherently safer, as they are less prone to overheating and explosion — a known issue with lithium-ion systems.
With the ability to function efficiently at room temperature and deliver stable performance across hundreds of cycles, magnesium batteries could soon power electric vehicles, grid-scale storage systems, and portable electronics — all while reducing reliance on scarce and environmentally taxing materials.
Toward the Next Generation of Battery Design
The researchers’ work also provides a new design philosophy for future cathode materials. The study highlights three key principles:
- Introducing structural free volume for easier ion transport.
- Controlling particle size at the nanoscale for optimal diffusion.
- Ensuring compatibility with advanced electrolytes that support magnesium ion movement.
These principles don’t just apply to magnesium — they could influence the development of other next-generation battery technologies, from sodium-ion to solid-state systems.
By focusing on structure and chemistry together, scientists are learning how to design materials that meet both performance and sustainability goals — a crucial balance in an era of growing environmental awareness.
The Promise and the Path Ahead
Of course, challenges remain before magnesium batteries can be mass-produced. Researchers still need to improve energy density, optimize electrolytes, and scale up production techniques. But the progress achieved by Tohoku University marks a pivotal step. It proves that magnesium batteries can operate efficiently under normal conditions, something long thought nearly impossible.
As Ichitsubo and his colleagues continue refining their design, industries around the world are watching closely. The potential payoff is immense — a battery technology that is faster, safer, cleaner, and cheaper than anything we use today.
A Future Built on Earth’s Abundance
In the grand story of human innovation, this breakthrough is more than a technical achievement — it’s a reminder that nature itself holds the answers to our most pressing challenges. While we once reached into remote deserts and salt flats in search of lithium, perhaps the future of energy lies much closer to home, in the magnesium-rich crust beneath our feet.
The rechargeable magnesium battery developed at Tohoku University is not just another scientific milestone; it is a symbol of possibility — that sustainability and progress need not be at odds, and that through creativity, precision, and perseverance, we can build a cleaner, more resilient world.
In every glowing LED powered by their prototype, we see a glimpse of tomorrow — one where light, power, and life are sustained not by scarcity, but by abundance.
More information: Tomoya Kawaguchi et al, Amorphous oxide cathode enabling room-temperature rechargeable magnesium batteries, Communications Materials (2025). DOI: 10.1038/s43246-025-00921-0