In the world of cellular biology, the endoplasmic reticulum (ER) is often depicted in textbooks as a compact and isolated structure near the nucleus, with an essential role in protein trafficking and secretion. However, this portrayal belies the true nature of the ER, which is far more dynamic, expansive, and integral to cell function than initially described. The ER spans much of the cell’s cytoplasm and establishes membrane contacts with a variety of organelles, helping to regulate crucial cellular processes such as fat and sugar metabolism and even immune responses. One of the key discoveries in recent research has been that pathogens can manipulate these ER interactions for their benefit, often hijacking fundamental cellular processes to promote their own replication and survival.
The laboratory of Rebecca Lamason at the Department of Biology at MIT has been at the forefront of these revelations. A study published by Lamason’s team in the Journal of Cell Biology made a groundbreaking discovery about Rickettsia parkeri, a bacterial pathogen that operates in the cytosol of host cells. The team found that R. parkeri establishes an extensive and stable interaction with the rough ER (rER), leading to previously unknown interkingdom contacts between a bacterial pathogen and a eukaryotic membrane. This research not only sheds light on bacterial infection mechanisms but also presents insights into novel therapeutic targets for infectious diseases.
A New Way to Study Rickettsia Infections
Rickettsia parkeri is studied as a model organism to understand more virulent species like Rickettsia rickettsii, the causative agent of Rocky Mountain Spotted Fever (RMSF), which can be lethal if left untreated. Infected ticks carry and transmit these bacteria, making RMSF a serious health concern in endemic regions of North America. Rickettsia species are considered obligate intracellular pathogens, meaning they cannot live outside living cells. This dependency makes Rickettsia species, including R. parkeri, difficult to study because they can only be cultured inside eukaryotic host cells. This presents unique challenges for scientists trying to dissect the fundamental interactions involved in their life cycle.
Despite the complexity of studying these pathogens, the Lamason Lab set out to investigate how R. parkeri spreads from one infected cell to another. The conventional model of Rickettsia infection is based on the idea that the bacteria spread between cells through specialized junctions that connect neighboring cells. Once at these junctions, the bacteria enter the neighboring cell through engulfment. Similar pathogens, like Listeria monocytogenes, have developed sophisticated mechanisms to force entry into adjacent cells by pushing themselves through the cytoplasm using an actin tail—a process known as actin-based propulsion. Rickettsia has been shown to form actin tails, but it loses them just before reaching the junction between cells. Nonetheless, the bacteria continue to spread.
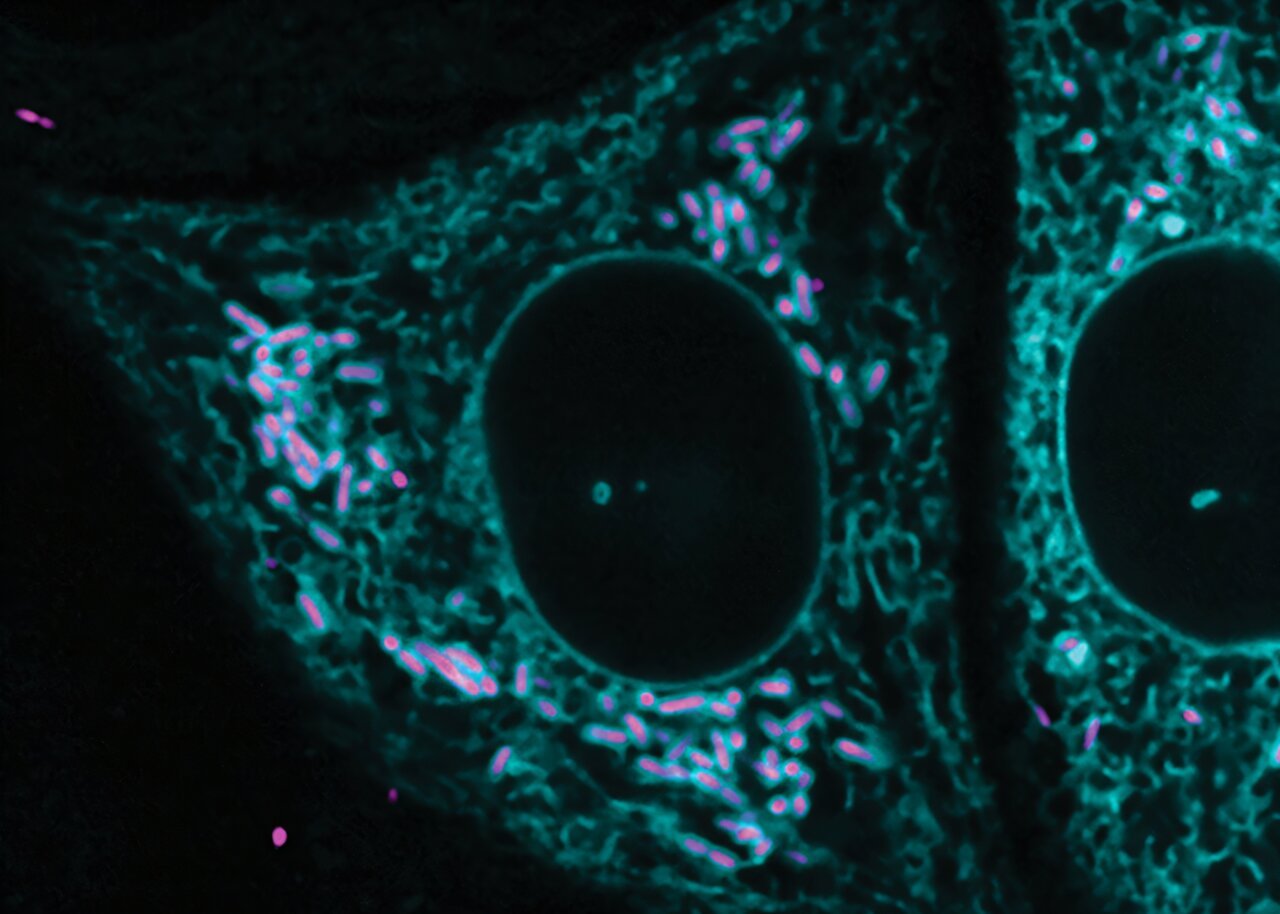
This anomaly sparked the curiosity of Yamilex Acevedo-Sánchez, then a graduate student and the first author of the paper. While attending a seminar about the ER’s lesser-known roles in cell biology, she hypothesized that R. parkeri might use the ER network to bridge the gap between cells during its infection cycle. Instead of finding the bacteria at cell junctions, Acevedo-Sánchez unexpectedly observed R. parkeri interacting with the ER in a previously unobserved fashion.
An Unforeseen Discovery: Rickettsia’s Interaction with the ER
Acevedo-Sánchez’s initial observations involved a cell line engineered to study how R. parkeri spreads. Instead of observing the expected interactions between the bacteria and cell junctions, she discovered an unusually high percentage of R. parkeri surrounded by the ER. This was a remarkable finding because the typical membrane contacts that help regulate interactions between organelles like the ER and the mitochondria are typically brief, lasting only a few seconds to minutes. These contacts often serve to facilitate the transfer of materials, such as lipids or calcium, between the organelles. However, what Acevedo-Sánchez observed in her experiments was much more stable—despite the rapid turnover typical of ER connections, the ER membranes appeared to wrap tightly around the bacteria and remain engaged.
Interestingly, the membrane contacts between the ER and the bacteria appeared to occur at a precise distance of about 55 nanometers, a gap consistent with the measurements of membrane contacts in eukaryotic cells. Upon ruling out the possibility that this was merely a part of the immune response, the researchers concluded that the ER’s proximity to the bacteria was genuine and biologically significant.
“I’m of the mind that if you want to learn new biology, just look at cells,” Acevedo-Sánchez remarked. The ability to manipulate cellular organelles and observe the way they form contact with bacterial pathogens, she realized, could provide deep insights into how infections work.
Collaborating to Unveil the Mystery
To further explore these unexpected findings, Acevedo-Sánchez sought the expertise of the Center for Nanoscale Systems at Harvard University, using advanced imaging techniques to gather more detailed data. Specifically, she employed focused ion beam scanning electron microscopy (FIB-SEM) to obtain higher-resolution images of the interactions between the ER and the R. parkeri bacteria. FIB-SEM is a cutting-edge technology that involves shooting a sample with a focused ion beam and taking sequential, high-resolution pictures as the beam removes thin layers of the cell sample. The resulting images can be analyzed to reconstruct 3D models of the interactions at the nanoscale.
The data collected from these advanced imaging techniques provided the team with thousands of images, which were analyzed with the help of machine learning algorithms. By marking areas in the images—such as the mitochondria, bacteria, and the ER—these algorithms could identify patterns and reconstruct three-dimensional models, offering a clearer and more complete view of what was happening at the molecular level.
Despite these efforts, Acevedo-Sánchez found that fewer than 5% of R. parkeri were involved in ER interactions. However, small interactions have a significant impact when it comes to infection processes. In the case of R. parkeri, this minimal interaction proved to be crucial. Specifically, the study found that the bacteria’s behavior depended on whether they were motile or nonmotile. While motile bacteria were able to form actin tails, it was the nonmotile bacteria—lacking these tails—that demonstrated a higher degree of interaction with the ER. When researchers created mutants that could no longer form actin tails, the number of bacteria interacting with the ER increased by twenty-five times. This revealed a clear connection between the bacteria’s mobility and their interactions with the host cell’s internal structures, which seemed to be exploited by the pathogen for its own propagation.
The Role of VAP Proteins in Infection
To investigate the molecular underpinnings of the ER-Rickettsia interaction, the team focused on the VAP proteins, which are known to play an essential role in enabling membrane contact between organelles. These proteins mediate the interaction between the ER and other organelles during various cellular processes, and they are often hijacked by pathogens to aid in their infection cycle. The Lamason Lab team discovered that during R. parkeri infections, these VAP proteins were recruited to the bacteria itself. When VAP proteins were knocked out, the frequency of interactions between the bacteria and the ER decreased, suggesting that R. parkeri uses the host’s mechanisms to control these membrane contacts during infection.
Though Acevedo-Sánchez has since taken up a position at AbbVie, a pharmaceutical company, the research on R. parkeri’s interactions with the ER continues to unfold in Lamason’s lab. This research remains central to understanding not only the life cycle of Rickettsia species but also the broader implications of organelle hijacking in pathogenic infection.
Future Directions and Unanswered Questions
In addition to the intriguing findings about R. parkeri and its manipulation of the ER, the Lamason Lab has begun exploring the connection between the ER and other organelles. Notably, mitochondria and bacteria are thought to have evolved from a common ancestor. Lamason and her team are now investigating whether R. parkeri could establish similar types of interactions with the mitochondria, as they are structurally similar to the ER.
While no direct proof has yet emerged that R. parkeri forms contacts like those established by mitochondria, these findings represent an exciting new avenue for future research. Given the stability of these membrane interactions between the ER and R. parkeri, Lamason believes that these interactions likely serve a purpose beyond random accidents.
“It’s not just bacteria bumping into the ER. These interactions are extremely stable,” Lamason observed. “The ER is clearly extensively wrapping around the bacterium, and it is still connected to the network. It seems like it has a purpose—what that purpose is remains a mystery.”
Conclusions
The groundbreaking research from the Lamason Lab has shown that R. parkeri interacts in a profound and previously unknown way with the host’s endoplasmic reticulum during infection. These stable membrane contacts represent a new example of interkingdom communication, demonstrating that bacteria are capable of manipulating cellular machinery to suit their needs. This discovery opens the door to new investigations into the role of organelle hijacking during pathogen infection, as well as its potential implications for developing more effective therapies for treating Rickettsia infections and other diseases caused by obligate intracellular pathogens.
In studying these types of pathogen-host interactions, science takes a crucial step forward in understanding the complex and dynamic cellular machinery of the human body—and perhaps in one day developing therapeutic approaches that can thwart such hijacking of fundamental cellular processes. The implications for this work extend far beyond R. parkeri, showing a new frontier in understanding the molecular battles fought between pathogens and cells during infection.
Reference: Yamilex Acevedo-Sánchez et al, Rickettsia parkeri forms extensive, stable contacts with the rough endoplasmic reticulum, Journal of Cell Biology (2025). DOI: 10.1083/jcb.202406122