In a breakthrough that touches the icy edge of physics, a Spanish professor has solved a century-old puzzle that stumped some of the greatest minds in science—including Albert Einstein.
In a recent paper published in The European Physical Journal Plus, José Martín-Olalla of the University of Seville has established, for the first time, a direct theoretical link between Nernst’s theorem—an early 20th-century observation about entropy at low temperatures—and the second law of thermodynamics, which governs the irreversible increase of entropy in the universe.
This might sound like an abstract reshuffling of dusty physics principles, but in reality, it marks a profound shift in our understanding of the deepest workings of nature. It revisits an argument first posed by Walther Nernst in 1905 and later dismissed by Einstein, and in doing so, it may reshape how thermodynamics is taught, interpreted, and understood for generations to come.
At the Edge of Zero: The Birth of a Theorem
In the early 1900s, scientists were just beginning to understand how matter behaves at temperatures nearing absolute zero—a chilling -273°C, where molecular motion essentially stops. Walther Nernst, working at the crossroads of chemistry and physics, observed something peculiar and consistent: as the temperature of a system approached absolute zero, the entropy exchanges—the measure of disorder or randomness in a system—tended to zero.
He formulated this into what we now call Nernst’s theorem, or sometimes the third law of thermodynamics. Nernst argued that if absolute zero were ever attainable, it would allow for the theoretical creation of a perfect engine—one that converts all heat into useful work with no waste. Such an engine would break the sacred second law of thermodynamics, which insists that disorder must always increase.
So, Nernst concluded: absolute zero must be fundamentally unreachable.
But then came Einstein.
Einstein’s Rebuttal: Separating the Laws
Einstein, never one to take even the sharpest logic at face value, raised an objection. He agreed that such a perfect engine was theoretically problematic—but insisted that it was impossible to construct such a device in practice. And because it couldn’t exist, he argued, it couldn’t serve as a foundation for proving anything about the second law. The impossibility of reaching absolute zero, then, must be a separate principle altogether—not an extension of entropy’s law, but an independent third principle.
That viewpoint stuck. For over a century, physicists have treated Nernst’s observation as an axiom of its own—floating near the edge of our physical understanding but isolated from the foundational laws that govern heat, work, and entropy.
Now, Martín-Olalla says that division was a mistake.
A Virtual Engine and the Return to Unity
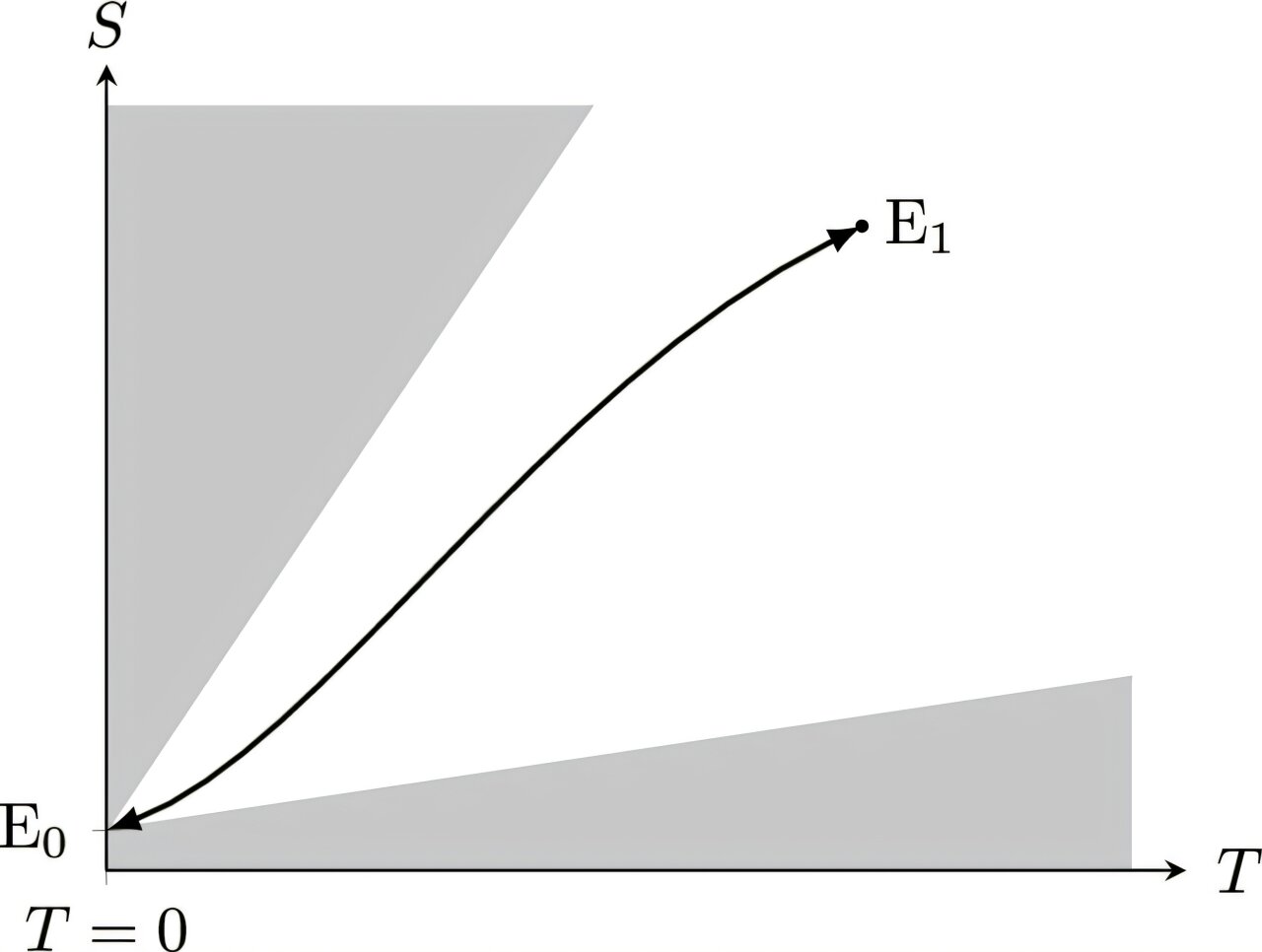
In his bold reanalysis, Martín-Olalla doesn’t rely on new experimental data or exotic technology. He revisits the very assumptions that framed the original argument—and finds that something crucial was missing all along.
Nernst imagined a machine that could violate the second law. Einstein responded that the machine was impossible. But Martín-Olalla adds a vital nuance: the machine was never meant to be real. It was, and should have always been understood as, a virtual construct—a thought experiment engine that neither consumes heat nor produces work.
In this light, the second law of thermodynamics doesn’t demand the machine be real. It only requires its theoretical presence to reason about the limits of entropy. And if the machine is understood as a mathematical fiction, not a physical object, then the logic Nernst used holds true—not in opposition to the second law, but as a direct consequence of it.
“When you approach the second law in its formal version,” Martín-Olalla explains, “you find that the engine Nernst imagined is not only compatible with the law—it’s required. But it must remain virtual. That changes everything.”
Rethinking Absolute Zero
This isn’t just a semantic twist—it fundamentally alters how physicists might conceptualize temperature itself.
As Martín-Olalla notes, we often think of hot and cold as sensations. Even when we study temperature scientifically, we rely on empirical markers: the pressure of a gas, the expansion of a metal rod. But none of those tools approach the theoretical clarity offered by entropy and the second law.
At absolute zero, entropy becomes unique—there is only one microstate, one configuration of matter, and no uncertainty remains. That uniqueness, Martín-Olalla argues, is what the second law demands. And the cancellation of specific heat, long treated as an independent quirk noted by Nernst in 1912, is merely an appendix—a corollary to the larger story.
Correcting Einstein?
In scientific circles, challenging Einstein is something done only with care, clarity, and exceptional evidence. Martín-Olalla does all three—not by tearing down Einstein’s work, but by reframing the debate.
Einstein correctly recognized that Nernst’s engine could not exist. But what Martín-Olalla shows is that the formal structure of thermodynamics never needed it to exist physically. It only needed the idea of it. Once that distinction is made, Nernst’s original conclusion—that absolute zero is unreachable, and that entropy exchanges vanish as temperature nears it—emerges naturally from the second law.
This reintegration eliminates the need to treat the third law as something entirely separate. It becomes a logical extension, rather than a separate assumption.
Changing the Classroom—And the Canon
As with all great theoretical breakthroughs, acceptance will take time. Martín-Olalla is candid about this.
“The students in the thermodynamics course I teach were the first to learn about this demonstration,” he said. “I hope that with this publication the demonstration will become better known, but I know that the academic world has a great deal of inertia.”
But every revolution begins with a few thoughtful readers. And in a world where scientific attention is often dominated by flashy particles and astronomical headlines, there’s something quietly profound about solving a puzzle that has lingered since the birth of modern physics.
It’s a reminder that science moves not only by leaps in technology, but also by shifts in understanding—that sometimes, to move forward, we must return to the foundations and look again, more carefully, at what we thought we knew.
With a virtual engine and a little imagination, Martín-Olalla may have just changed the way we think about cold, entropy, and the laws of the universe. And he did it not by breaking the rules—but by showing how they were always meant to work.
Reference: Jos-María Martín-Olalla, Proof of the Nernst theorem, The European Physical Journal Plus (2025). DOI: 10.1140/epjp/s13360-025-06503-w