Imagine a world where particles can be in two places at once, where simply observing something can change its reality, and where randomness—not certainty—governs the rules of nature. This is not science fiction. This is quantum mechanics, the most successful and mind-bending theory in all of physics. It underpins modern technology, fuels our understanding of the cosmos, and challenges our perception of reality.
Yet, quantum mechanics is often portrayed as mysterious or incomprehensible. Particles behaving like waves? Cats that are both dead and alive? While these ideas sound fantastical, they are grounded in rigorous mathematics and decades of experimentation. In this article, we’ll strip away the confusion and explore the key concepts of quantum mechanics in simple, engaging language—without sacrificing depth or wonder. Our journey will take us from the smallest particles in nature to the frontiers of human understanding.
Classical Physics: The World Before Quantum
Before quantum mechanics, the universe made perfect sense—at least to classical physicists. Isaac Newton’s laws described how planets move, how apples fall, and how forces interact. In the 19th century, scientists believed that if you knew everything about a system—its position, velocity, and the forces acting upon it—you could predict its future with absolute certainty. This is known as determinism.
Light was understood as a wave, electricity and magnetism were unified by James Clerk Maxwell’s equations, and the atomic theory of matter was gaining acceptance. It seemed the laws of nature were close to being fully uncovered.
But cracks began to appear at the dawn of the 20th century. When scientists started investigating very small things—like atoms and subatomic particles—they discovered behavior that defied Newtonian expectations. Light acted like a particle. Electrons behaved like waves. And some phenomena could only be explained if energy came in discrete chunks—quanta.
These strange discoveries would give birth to a new kind of physics. One that is not deterministic but probabilistic. Not intuitive, but precise. Welcome to quantum mechanics.
The Birth of Quantum Theory: Energy Comes in Quanta
The story of quantum mechanics begins with a problem in thermal radiation. Scientists studying how objects emit heat—like a piece of iron glowing red-hot—noticed something odd. According to classical physics, a hot object should emit infinite energy at short wavelengths. This was known as the ultraviolet catastrophe.
In 1900, German physicist Max Planck proposed a radical solution. He suggested that energy is not continuous, but comes in tiny packets called quanta. He introduced a constant—now known as Planck’s constant—to describe the size of these energy chunks. This seemingly simple idea turned the tide of physics and marked the birth of quantum theory.
Planck’s formula matched experimental data perfectly. But it also implied that the energy of oscillating atoms could only take on specific, discrete values—not any value at all. The smooth continuum of classical physics had shattered.
In 1905, Albert Einstein extended Planck’s idea to light itself. He proposed that light could be thought of as particles, called photons, each carrying a quantum of energy. This explained the photoelectric effect, where light striking a metal surface knocks electrons free—a phenomenon classical physics couldn’t explain.
Einstein’s theory showed that light was both a wave and a particle. It was the first glimpse into the quantum duality that defines the microcosmic world.
Wave-Particle Duality: When Particles Behave Like Waves
One of the most astonishing aspects of quantum mechanics is wave-particle duality—the idea that particles like electrons and photons can behave like waves, and waves like particles.
In 1924, a young French physicist named Louis de Broglie suggested that if light (a wave) can act like a particle, then maybe particles (like electrons) can act like waves. His hypothesis was confirmed by experiments showing that electrons fired through a crystal created interference patterns—just like ripples on a pond. Electrons, in other words, were not just particles—they were also waves.
This was a revolutionary insight. A single electron could interfere with itself, as if it were traveling along multiple paths simultaneously. The classic experiment demonstrating this is the double-slit experiment.
When electrons are fired at a barrier with two slits and allowed to strike a screen behind, they form an interference pattern—indicating wave-like behavior. But when scientists observe which slit the electron goes through, the pattern vanishes, and the electrons behave like particles. Observation collapses the wave-like possibilities into a single outcome.
This is not just a trick of measurement—it’s a fundamental feature of nature. Observation affects reality. The electron seems to “decide” how to behave based on whether it is being watched.
The Uncertainty Principle: Limits to Knowledge
In classical physics, knowing the position and velocity of a particle lets you predict its future. But in the quantum world, this certainty vanishes.
In 1927, German physicist Werner Heisenberg formulated the Uncertainty Principle. It states that the more precisely you know a particle’s position, the less precisely you can know its momentum (mass times velocity), and vice versa. This is not a limitation of instruments—it’s a fundamental property of nature.
At the heart of the uncertainty principle is the wave nature of particles. A perfectly known position means an undefined wavelength (and therefore momentum), while a well-defined momentum means the wave spreads out in space, making position uncertain.
This built-in fuzziness means the quantum world is not deterministic. Even with complete information, we can only calculate probabilities of outcomes—not certainties.
The Schrödinger Equation: The Engine of Quantum Mechanics
Just as Newton’s laws describe motion in classical physics, quantum mechanics has its own fundamental law: the Schrödinger equation. Developed by Austrian physicist Erwin Schrödinger in 1926, this equation governs how quantum systems evolve over time.
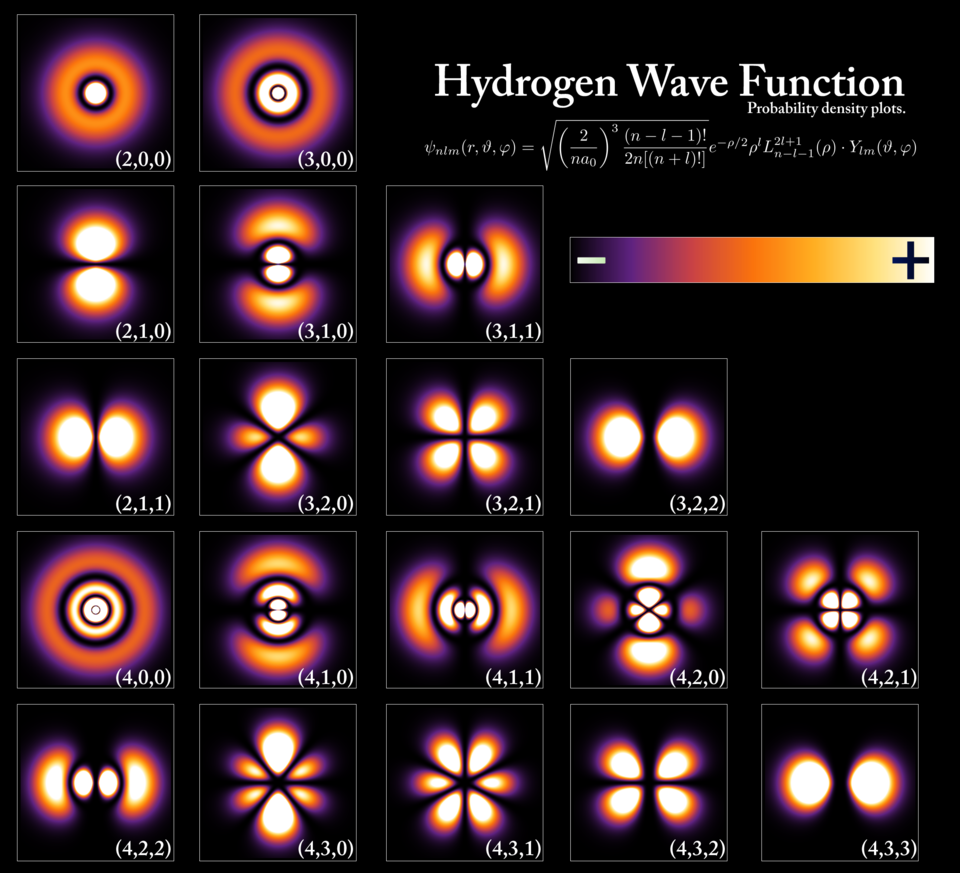
The Schrödinger equation doesn’t give the position of a particle directly. Instead, it provides a wavefunction—a mathematical function that contains all possible information about a system. The square of the wavefunction’s amplitude at any point gives the probability of finding the particle there.
For example, if you use the Schrödinger equation to describe an electron in an atom, you find that it doesn’t orbit the nucleus like a planet. Instead, it exists as a cloud of probability—a blurry region where the electron is likely to be found.
The Schrödinger equation beautifully predicts the behavior of electrons, atoms, and molecules. It lies at the heart of quantum chemistry and explains the structure of the periodic table, the formation of chemical bonds, and even the colors of the stars.
Quantum Superposition: All States at Once
One of the strangest and most powerful principles of quantum mechanics is superposition. A quantum system can exist in multiple states at once—until it is measured.
A famous thought experiment illustrating this is Schrödinger’s cat. Imagine a cat in a sealed box with a radioactive atom, a Geiger counter, a vial of poison, and a hammer. If the atom decays, the counter detects it, the hammer breaks the vial, and the cat dies. If not, the cat lives. According to quantum mechanics, until the box is opened, the atom is in a superposition of decayed and not decayed—and so the cat is both dead and alive.
This sounds absurd, but it mirrors real experiments on particles, which remain in superposition until measured. Superposition is real—and it’s at the heart of quantum computing, where bits (qubits) can be in both 0 and 1 states simultaneously, enabling massively parallel processing.
Quantum Entanglement: Spooky Action at a Distance
Another bizarre and fascinating feature of quantum mechanics is entanglement. When two particles become entangled, their states are linked—even when separated by vast distances.
If you measure the state of one entangled particle, the other’s state is instantly determined, no matter how far apart they are. Einstein famously called this “spooky action at a distance,” because it seemed to violate the speed of light limit for information transfer.
But experiments have confirmed entanglement time and again. It doesn’t involve faster-than-light communication, but rather a deep connection in the quantum fabric of reality. Entanglement is more than a curiosity—it’s a key resource in quantum teleportation, quantum encryption, and the emerging field of quantum networks.
Quantum Tunneling: Passing Through the Impossible
In classical mechanics, if a particle doesn’t have enough energy to climb a barrier, it stops. In quantum mechanics, it might tunnel through it.
Quantum tunneling occurs because particles are described by wavefunctions that spread out in space. If the wavefunction extends through a barrier, there’s a nonzero probability the particle will appear on the other side—even if classically impossible.
Tunneling is essential to many physical phenomena. It powers the nuclear fusion that lights the Sun. It’s the principle behind scanning tunneling microscopes, which image atoms on surfaces. And it’s used in modern electronics, including flash memory and tunnel diodes.
The Measurement Problem: What Does It Mean to Observe?
Quantum mechanics tells us how systems evolve and what probabilities to expect—but it doesn’t explain how or why a single outcome emerges when we measure something. This is known as the measurement problem.
Why does observing a system collapse its wavefunction into a definite state? What counts as an observation? Is consciousness required? Or is it simply interaction with the environment?
Different interpretations of quantum mechanics offer different answers. The Copenhagen interpretation, favored by Bohr and Heisenberg, holds that wavefunction collapse is a real, physical process triggered by measurement. The Many-Worlds interpretation says all possible outcomes occur, each in a separate universe. The pilot-wave theory, proposed by de Broglie and Bohm, adds hidden variables to restore determinism.
No interpretation has been definitively proven, and the debate remains open. But all lead to the same experimental predictions—and all point to a universe more subtle and mysterious than classical physics ever imagined.
Quantum Mechanics and Modern Technology
Quantum mechanics isn’t just theoretical—it powers much of our modern world. Lasers, transistors, LEDs, MRI machines, atomic clocks, and computer chips all rely on quantum principles.
Quantum theory also drives new frontiers of technology. Quantum computing aims to solve problems beyond the reach of classical computers, using superposition and entanglement. Quantum cryptography offers theoretically unbreakable encryption. Quantum sensors can detect gravitational waves, magnetic fields, and molecular signatures with unprecedented sensitivity.
These technologies promise to revolutionize medicine, communications, finance, and security—and they all rest on the weirdness of quantum mechanics.
Quantum Mechanics and the Universe
Quantum mechanics isn’t confined to the lab—it’s woven into the cosmos. It explains the formation of atoms in the early universe, the behavior of stars, and the emission of radiation from black holes (via Stephen Hawking’s theory of Hawking radiation).
It also merges with Einstein’s theory of relativity in the field of quantum field theory, which describes the fundamental forces and particles of nature. The Standard Model of particle physics is a quantum theory that explains almost everything we observe—except gravity.
Unifying quantum mechanics and general relativity remains one of the great challenges of physics. Approaches like string theory and loop quantum gravity attempt this unification, but a complete theory of quantum gravity remains elusive.
Conclusion: The Quantum Frontier
Quantum mechanics is strange, beautiful, and profoundly accurate. It has revealed a universe that defies common sense, yet obeys elegant mathematical laws. It shows us that reality is not fixed but probabilistic, not objective but relational, not classical but quantum.
Despite its counterintuitive nature, quantum mechanics has stood the test of time. It’s been confirmed by countless experiments and has revolutionized both our understanding of nature and our technological capabilities.
And yet, mysteries remain. What is the true nature of the wavefunction? Can we unify quantum mechanics with gravity? What lies at the quantum roots of spacetime?
As we continue to probe the quantum world, we may not only unlock new technologies but also reshape our understanding of reality itself. One thing is certain: the quantum journey is far from over, and the best may be yet to come.