For nearly a century, scientists have pursued a fundamental mystery: how cancer cells reprogram their energy use to fuel unchecked growth. This quest has taken them deep inside the cell—to the biochemical border between the cytosol and mitochondria, where pyruvate, a critical product of glucose metabolism, awaits its fate. Does it feed the mitochondrial furnace, powering life itself through oxidative phosphorylation? Or does it linger in the cytosol, channeled instead into the fermentation-like frenzy that marks tumorigenic metabolism?
Now, researchers at Duke University Medical Center and Wake Forest University School of Medicine have added a surprising new player to this metabolic drama. In a groundbreaking study published in Nature Cell Biology, they’ve identified ALDH4A1, a mitochondrial enzyme historically associated with proline metabolism, as a third structural component of the mitochondrial pyruvate carrier (MPC) complex. Far from being a mere metabolic bystander, ALDH4A1 appears to function as a critical gatekeeper of mitochondrial pyruvate import—an essential process not just for cellular respiration but also for suppressing the metabolic chaos of cancer.
This revelation doesn’t just revise our understanding of mitochondrial biology—it could open up an entirely new front in the war on cancer.
The Warburg Enigma: Metabolism Meets Mutation
To appreciate the significance of this finding, one must first understand the metabolic idiosyncrasy that has long puzzled biologists: the Warburg effect. First described by Otto Warburg in the 1920s, this phenomenon refers to cancer cells’ tendency to favor aerobic glycolysis—a form of metabolism in which glucose is converted to lactate, even when oxygen is abundant.
On the surface, this seems inefficient. Oxidative phosphorylation in mitochondria yields far more ATP per molecule of glucose than glycolysis. Why would a rapidly growing cancer cell settle for less?
As it turns out, the benefits of glycolysis go beyond energy. The intermediates produced can be diverted into pathways that synthesize lipids, nucleotides, and amino acids—building blocks for cell growth. Meanwhile, the mitochondria, often seen as powerhouses, double as biosynthetic factories. This complex dance of energy and biosynthesis is choreographed by tightly regulated transport of molecules like pyruvate across mitochondrial membranes.
Here lies the crux of the discovery: control over mitochondrial pyruvate import is a metabolic switch. And ALDH4A1 appears to have its hand on the lever.
The Gate: Mitochondrial Pyruvate Carrier (MPC)
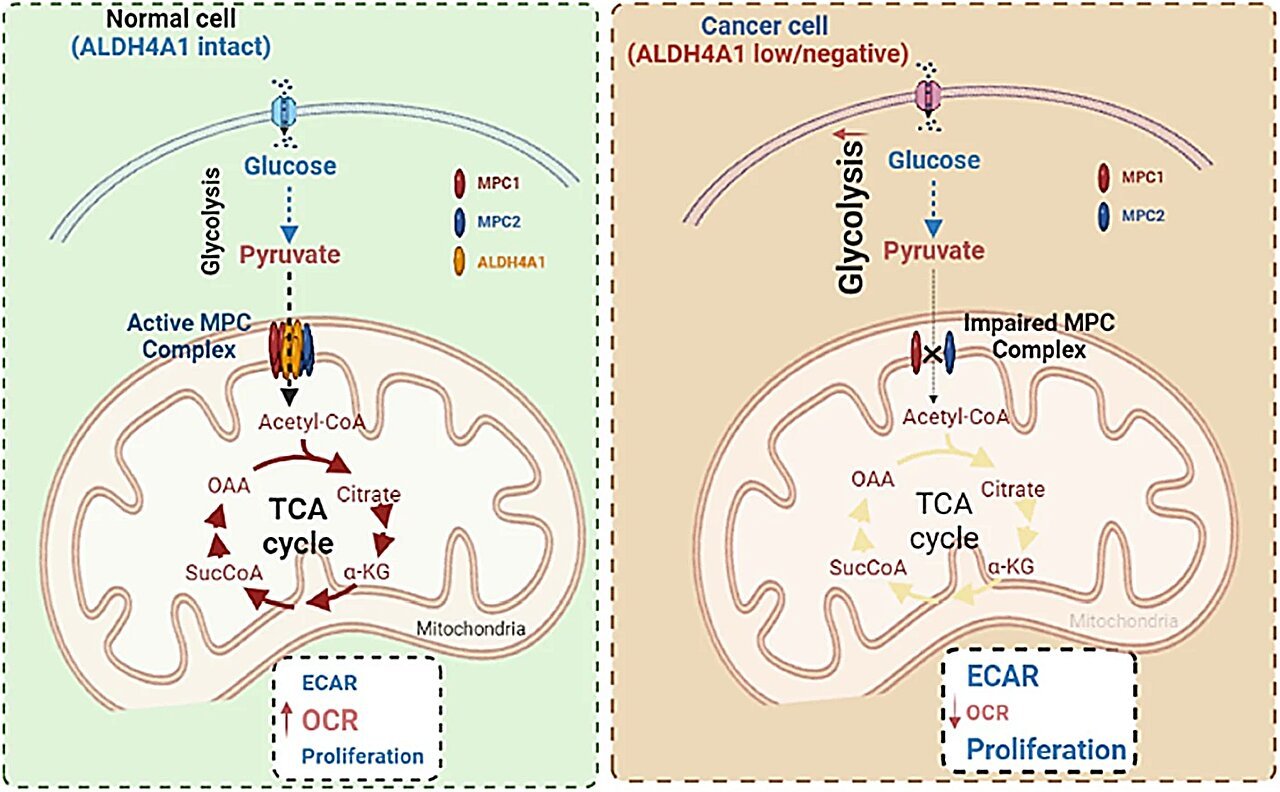
The mitochondrial pyruvate carrier, composed of two proteins—MPC1 and MPC2—was discovered relatively recently in 2012, solving a decades-old mystery of how pyruvate enters the mitochondrial matrix. This import step is vital. Without it, pyruvate cannot be converted into acetyl-CoA, the molecule that kicks off the tricarboxylic acid (TCA) cycle, also known as the Krebs cycle.
MPC1 and MPC2 work together as a dimeric transporter embedded in the inner mitochondrial membrane. Their role as gatekeepers of mitochondrial respiration has been well-established, but their regulation—how their structure, activity, and integrity are maintained—has remained a frontier.
Enter ALDH4A1, a gene encoding Delta-1-pyrroline-5-carboxylate dehydrogenase, long known for its role in proline catabolism. The enzyme catalyzes the oxidation of pyrroline-5-carboxylate to glutamate in mitochondria. It was not expected to have anything to do with pyruvate.
And yet, in a twist worthy of cellular mystery novels, researchers discovered that ALDH4A1 is not just nearby the MPC complex—it’s part of it.
An Unexpected Triad: ALDH4A1 Joins the MPC Complex
The study’s lead authors used co-immunoprecipitation, mass spectrometry, and blue-native polyacrylamide gel electrophoresis (PAGE) to map the physical interactions of proteins in the inner mitochondrial membrane. They found that ALDH4A1 co-purifies with MPC1 and MPC2, forming a stable trimeric complex. Importantly, this was not an incidental interaction. ALDH4A1 structurally integrated with the MPC dimer, creating a tripartite architecture that maintained the complex’s functional integrity.
When researchers knocked down ALDH4A1 expression, the consequences were profound. Pyruvate import into mitochondria declined significantly. Oxygen consumption rates dropped, indicating impaired mitochondrial respiration. Cytosolic pyruvate accumulated, rerouting metabolism toward glycolysis—a hallmark of the Warburg effect. In this altered metabolic state, cancer cells grew faster, migrated more aggressively, and even exhibited anchorage-independent growth, a key indicator of malignancy.
These in vitro results were supported by in vivo evidence. When human cancer cells with ALDH4A1 knockdown were implanted into nude mice, tumors grew larger and faster than controls. Conversely, overexpressing ALDH4A1 inhibited tumor growth, underscoring its tumor-suppressive function.
The Role of ALDH4A1: More Than Metabolism
The discovery that ALDH4A1 stabilizes the MPC complex and promotes mitochondrial pyruvate import positions it as a metabolic tumor suppressor. But the story doesn’t end with transport mechanics. The researchers also explored the drug-targeting potential of the ALDH4A1-MPC interaction.
Treatment with UK5099, a known MPC inhibitor, disrupted the association between ALDH4A1 and the MPC complex, while leaving MPC1/2 dimers intact. This suggests a functional interface between ALDH4A1 and the transporter dimer—an interaction that may be selectively druggable without wholly abolishing mitochondrial respiration.
Why is this important? Broad-spectrum MPC inhibition can be toxic, as healthy cells also rely on mitochondrial respiration. But if cancer cells depend more heavily on disrupted ALDH4A1 function to sustain their glycolytic bias, then targeting this interaction could offer metabolic precision therapy.
ALDH4A1 and Proline Metabolism: A Hidden Link to Cancer?
Although the new study focuses on ALDH4A1’s role in the MPC complex, it’s important to remember that this enzyme’s canonical function lies in proline degradation. This raises a fascinating question: is there a deeper link between proline metabolism and cancer?
The answer may be yes. Proline metabolism has long been associated with redox balance, collagen turnover, and tumor stroma interactions. Proline-derived glutamate can feed into the TCA cycle or be used for biosynthesis. Some studies have implicated proline dehydrogenase (PRODH) and related enzymes in both tumor suppression and promotion, depending on the context.
ALDH4A1’s dual role—processing proline while stabilizing MPC—suggests it may act as a metabolic node, integrating amino acid catabolism with central carbon metabolism. Its activity might tip the balance between respiration and glycolysis, between growth and quiescence, between malignancy and metabolic order.
Implications for Cancer Therapy: A Mitochondrial Achilles’ Heel?
The identification of ALDH4A1 as a critical component of the MPC complex offers new hope for targeting cancer metabolism. Traditional therapies have often focused on killing rapidly dividing cells, but tumors are notoriously adaptable. Their plasticity—especially in metabolism—is a key survival trait.
By re-routing energy production away from mitochondria, cancer cells evade metabolic checkpoints. Restoring mitochondrial pyruvate import, or targeting cells that block it, could force tumors back into a vulnerable metabolic state.
Moreover, drugs that modulate ALDH4A1 activity or its interaction with MPC may offer a selective metabolic vulnerability. Tumors that suppress ALDH4A1 to boost glycolysis might become addicted to this suppression. Inhibiting glycolysis or reactivating ALDH4A1 could trigger metabolic catastrophe.
This concept, known as synthetic lethality, has already been applied in other contexts (e.g., BRCA mutations and PARP inhibitors). Could ALDH4A1 become the center of a new synthetic-lethal strategy?
Beyond Cancer: The Broader Significance of MPC Regulation
While cancer has rightly taken center stage in this study, the implications of ALDH4A1’s role in mitochondrial metabolism go much further. Mitochondrial dysfunction is a hallmark not only of cancer but also of neurodegenerative diseases, metabolic disorders, and aging.
Understanding how MPC complexes are assembled and regulated could inform therapies for diseases like Parkinson’s, Alzheimer’s, diabetes, and cardiomyopathy. Pyruvate is more than a substrate—it is a nexus point for the cell’s decision to build, burn, or break down.
Future studies may explore how ALDH4A1’s structural role in the MPC complex is influenced by cell type, stress conditions, or nutrient availability. Does the trimeric complex adapt in neurons under oxidative stress? Do stem cells modulate MPC assembly during differentiation?
The answers may transform how we view mitochondrial biology—not as a static factory, but as a flexible metabolic network, dynamically rewired through structural proteins like ALDH4A1.
The Next Frontier: Toward Targeted Metabolic Rewiring
In a time when cancer treatment is rapidly moving toward precision medicine, metabolic interventions have often lagged behind. Yet, this study suggests that metabolism isn’t just a backdrop for oncogenes and tumor suppressors—it’s a driver of malignancy, and perhaps a lever for its control.
The revelation that a long-overlooked proline enzyme structurally integrates with the mitochondrial pyruvate carrier reshapes our understanding of metabolic gatekeeping. ALDH4A1 doesn’t just process amino acids—it stands watch over one of the most fundamental decisions in cellular life: where pyruvate goes.
By manipulating this gatekeeper, scientists may soon gain the power to rewire cancer metabolism from the inside out.
Author’s Note: The discovery of ALDH4A1’s role in the MPC complex represents a conceptual breakthrough with real translational potential. As our understanding of mitochondrial biology deepens, such studies pave the way for a new era of metabolism-focused therapies—precise, potent, and rooted in the cell’s own energetic logic.
Reference: Che-Chia Hsu et al, ALDH4A1 functions as an active component of the MPC complex maintaining mitochondrial pyruvate import for TCA cycle entry and tumour suppression, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01651-8