The human brain, with its hundred billion neurons and unfathomable complexity, is our most prized organ—and perhaps also our most fiercely guarded. Nestled inside the skull and sheathed in a fortress of cellular defenses, the brain is encased by the blood-brain barrier (BBB), a network of tightly packed endothelial cells that line the tiny capillaries weaving through the brain.
This barrier is one of evolution’s masterpieces. It filters the blood supplying the brain, blocking out potentially harmful pathogens and toxins. But like many great fortifications, the BBB is indiscriminate in its vigilance. Along with bacteria and viruses, it also bars life-saving therapeutics—gene therapies, antibodies, and even small molecule drugs—from reaching their intended targets in the brain.
For decades, this presented a frustrating paradox for neurologists and neuroscientists: many neurological disorders—from Alzheimer’s and Parkinson’s to glioblastoma and ALS—could potentially be treated with precision medicine approaches, if only those therapies could cross the BBB. Instead, treatments often fell short, diverted away at the very gates of the brain’s defenses.
Now, an unlikely ally may be stepping in to help: nitrous oxide, better known as “laughing gas.” In a remarkable new study published in Gene Therapy, researchers at UT Southwestern Medical Center demonstrated that this familiar anesthetic agent—when paired with focused ultrasound (FUS)—can dramatically enhance the safe, temporary opening of the BBB in mice. The implications are vast, potentially paving the way for transformative new treatments in neurology and neuropsychiatry.
From Dental Chair to Neurosurgical Innovation
Nitrous oxide has long been used in operating rooms and dental offices as a fast-acting, short-lived sedative and analgesic. It offers calming effects and mild euphoria but wears off quickly, making it a staple of outpatient care. But its role in the new study had nothing to do with its effects on pain or mood. Rather, its contribution was mechanical—something akin to what it does to balloon-shaped microbubbles coursing through the bloodstream.
Microbubbles are the linchpin of the FUS technique to open the BBB. Administered intravenously, these gas-filled spheres oscillate when struck with ultrasonic waves focused on a specific region of the brain. This oscillation disrupts the tight junctions of the endothelial cells, temporarily increasing permeability and allowing therapeutic agents to cross into the brain parenchyma.
But there’s a problem: in conventional protocols, the amount of microbubbles and the intensity of ultrasound needed to open the BBB pose safety risks. High-pressure FUS or excessive microbubble concentrations can damage delicate neural tissue, leading to hemorrhage or inflammation. As a result, the field has been searching for ways to reduce the mechanical burden of BBB opening without sacrificing efficacy.
That’s where nitrous oxide enters the story.
A Serendipitous Synergy
Led by Dr. Bhavya R. Shah, an Associate Professor of Radiology and Neurological Surgery at UT Southwestern, and first author Dr. Deepshikha Bhardwaj, the research team hypothesized that inhaling nitrous oxide might influence the properties of microbubbles during FUS. This idea wasn’t entirely new—scientists have known for years that different gases affect the size and longevity of microbubbles. Nitrous oxide, in particular, tends to diffuse into gas-filled cavities, making bubbles swell.
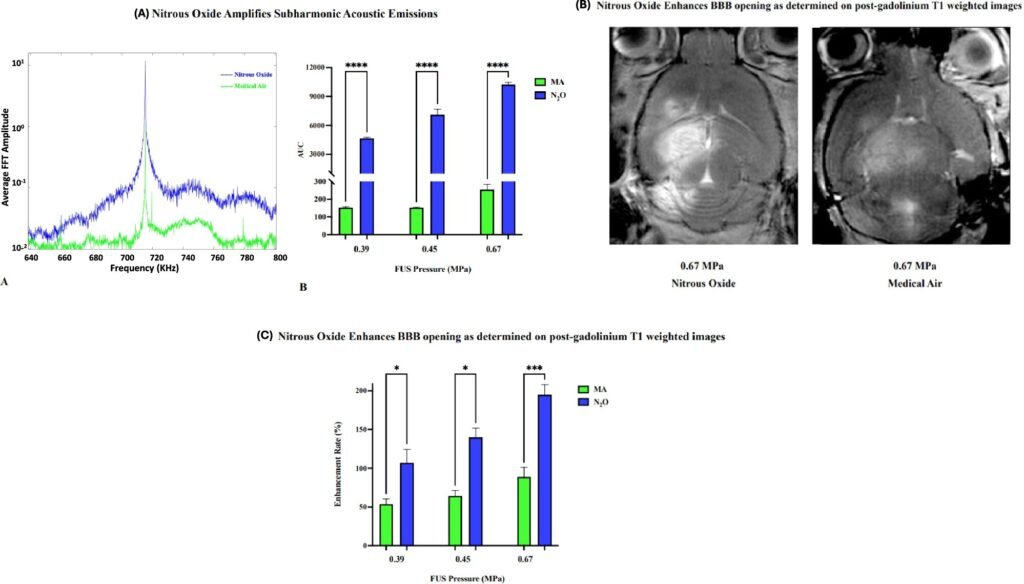
The researchers proposed a simple, yet profound intervention: switch the breathing gas during FUS from regular medical air to nitrous oxide. The team then tested the hypothesis in a mouse model, focusing ultrasound on specific brain regions while mice inhaled either air or nitrous oxide. The results were dramatic.
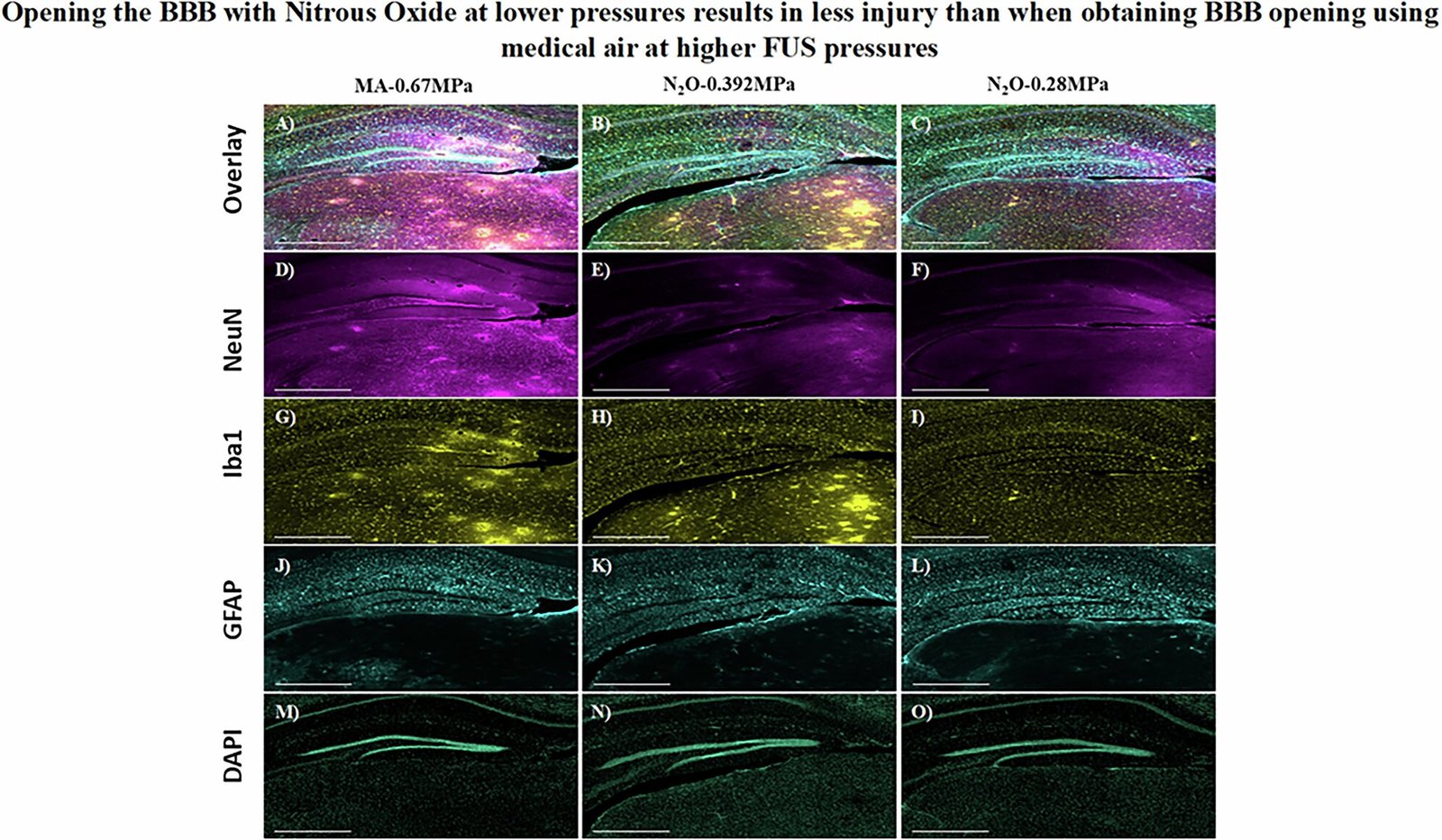
Mice breathing nitrous oxide required 1,000 times fewer microbubbles and significantly lower ultrasound pressure to achieve BBB opening compared to those breathing air. Not only did this lower the risk of tissue damage, but it also enhanced the efficiency of therapeutic delivery. To test the real-world impact of their approach, the team used a gene encoding green fluorescent protein (GFP), a classic molecular biology marker that glows under certain light.
In mice that received the gene therapy while breathing nitrous oxide, the targeted brain regions lit up with a vivid green glow—proof of successful gene delivery across the previously impermeable BBB.
The Promise and Power of FUS
To understand why this discovery is so meaningful, it helps to appreciate how focused ultrasound is redefining neurosurgery and neurotherapeutics. FUS is already being explored in human clinical trials for everything from brain tumors and Parkinson’s disease to depression. It offers a non-invasive, image-guided way to target specific areas of the brain with millimeter precision.
In traditional neurosurgery, access to deep brain structures often requires opening the skull, a process fraught with risks like infection and hemorrhage. In contrast, FUS can reach these same areas through the intact skull, guided by MRI. When combined with microbubbles, it becomes a powerful method to transiently open the BBB in a controlled, spatially restricted way.
But up until now, FUS carried its own limitations. To open the BBB reliably, researchers had to use high concentrations of microbubbles and push ultrasound parameters close to safety thresholds. This created a narrow therapeutic window, particularly for human applications. By introducing nitrous oxide into the protocol, Shah’s team has potentially widened that window—making FUS safer, more effective, and more adaptable to clinical translation.
Why Opening the BBB Matters
Neurological diseases have long lagged behind other fields in the gene therapy revolution. In part, this is because the brain is not easily accessible for delivery of genetic payloads—vectors like adeno-associated viruses (AAVs) are unable to cross the BBB in meaningful amounts. High doses are often needed, increasing the risk of immune reactions and off-target effects in other organs.
Imagine if we could instead target a specific brain region—say, the hippocampus in Alzheimer’s disease, or the striatum in Huntington’s—open the BBB locally for a brief window, and deliver a precise therapeutic gene directly where it’s needed. That is the vision this study moves us closer toward.
Beyond gene therapy, this approach could also enhance delivery of monoclonal antibodies for neurodegenerative diseases, chemotherapeutic agents for brain cancers, or even neuromodulatory peptides for treatment-resistant depression.
And because nitrous oxide is already widely used and FDA-approved for other purposes, its path to clinical integration may be much smoother than a new drug or novel material.
A Safety Advantage with Big Implications
Safety is always a primary concern in the brain. The beauty of the nitrous oxide-FUS synergy lies in the way it lowers mechanical stress on the tissue. Less ultrasound energy and fewer bubbles mean less cavitation—a term that describes the rapid expansion and collapse of microbubbles, which can cause microbleeds or inflammation if uncontrolled.
By enabling BBB opening at dramatically lower thresholds, nitrous oxide could help unlock the full potential of FUS while preserving the integrity of delicate brain structures. This becomes especially crucial when considering chronic diseases where repeat treatments are necessary.
The researchers were also careful to validate their findings across different brain regions and with different vectors. Importantly, they confirmed that the BBB resealed naturally within hours after treatment, as is typical with FUS-based methods.
A Glowing Gene and a Green Light for the Future
While the study’s primary outcome was technical—showing enhanced BBB permeability—it also carried a symbolic payload. The glowing green protein delivered into the mouse brain is more than a molecular marker. It’s a beacon of what may be possible in human neuroscience: controlled, safe, and reversible delivery of complex therapies into the brain.
As Dr. Shah emphasized, this work is still at the preclinical stage. Before the technique can be used in people, researchers will need to conduct extensive safety studies and clinical trials. But the early data are promising, and the team is already laying the groundwork for human translation.
What’s particularly exciting is the generalizability of the approach. It’s not tied to a specific disease or vector. In theory, any condition that could benefit from precise delivery of therapeutic molecules into the brain could be a candidate—whether genetic, oncologic, infectious, or psychiatric.
Breaking Barriers—Literally and Figuratively
Science often advances in fits and starts—periods of incremental progress punctuated by sudden leaps. The UT Southwestern study may represent one such leap. By marrying two familiar technologies—FUS and nitrous oxide—the researchers have reimagined a fundamental barrier in neurotherapeutics.
It’s a reminder that innovation doesn’t always require inventing something new. Sometimes, it involves seeing an old tool in a new light, combining existing knowledge in creative ways, and having the courage to ask: What if we tried this?
And in doing so, we edge closer to solving one of the most persistent puzzles in medicine: how to treat the brain as effectively as we treat the rest of the body.
What Comes Next?
As of now, the UT Southwestern team is preparing for the next stage: translational studies and clinical trial planning. The immediate goals include:
- Validating the safety of nitrous oxide-enhanced BBB opening in larger animals.
- Testing the delivery of therapeutic genes or antibodies relevant to human diseases.
- Assessing whether repeated sessions of BBB opening remain safe over time.
- Exploring other inhaled gases that might modulate microbubble dynamics similarly or even better.
If successful, clinical trials could follow within a few years, potentially marking a new era in how neurologists and neurosurgeons deliver care.
The Dawn of a New Neuroscience Frontier
The blood-brain barrier is no longer the impenetrable wall it once seemed to be. With precise, localized, and reversible techniques like focused ultrasound—now supercharged by the humble powers of nitrous oxide—we stand at the threshold of a new era in brain medicine.
It’s not often that a substance best known for calming dental patients ends up revolutionizing gene therapy. But science has a long history of surprises, and this may be one of the most promising yet.
In the not-too-distant future, it’s conceivable that patients with Alzheimer’s, glioblastoma, or intractable epilepsy will walk into a clinic, breathe in a little gas, lie back under an ultrasound scanner—and receive a treatment that was once considered impossible.
And all because someone dared to wonder: What happens if we make the brain laugh a little first?
Reference: Deepshikha Bhardwaj et al, Nitrous oxide enhances MR-guided focused ultrasound delivery of gene therapy to the murine hippocampus, Gene Therapy (2025). DOI: 10.1038/s41434-025-00530-z