In the world of cancer research, every discovery is a race against time. Melanoma, the most lethal form of skin cancer, has long outpaced many efforts to stop it. But a new study has found something extraordinary: melanoma’s most aggressive forms may be feeding their own destruction.
Scientists at Lund University in Sweden have uncovered that malignant melanoma cells are overactivating two essential energy-producing processes deep within the cell’s mitochondria—the biological engines that keep cells alive and working. Their findings, now published in the journal Cancer, show that when these hyperactive mitochondrial systems are disrupted, melanoma cells falter and die.
And even more striking: this can be done with drugs we already have.
Inside the Cell’s Hidden Machinery
To understand this breakthrough, it helps to zoom in—not just with a microscope, but with imagination. Picture a city inside each of your cells. Roads and highways carry information. Police regulate checkpoints. But at the heart of it all, power plants—the mitochondria—keep everything running.
In aggressive melanoma tumors, researchers discovered, these power plants are running on overdrive.
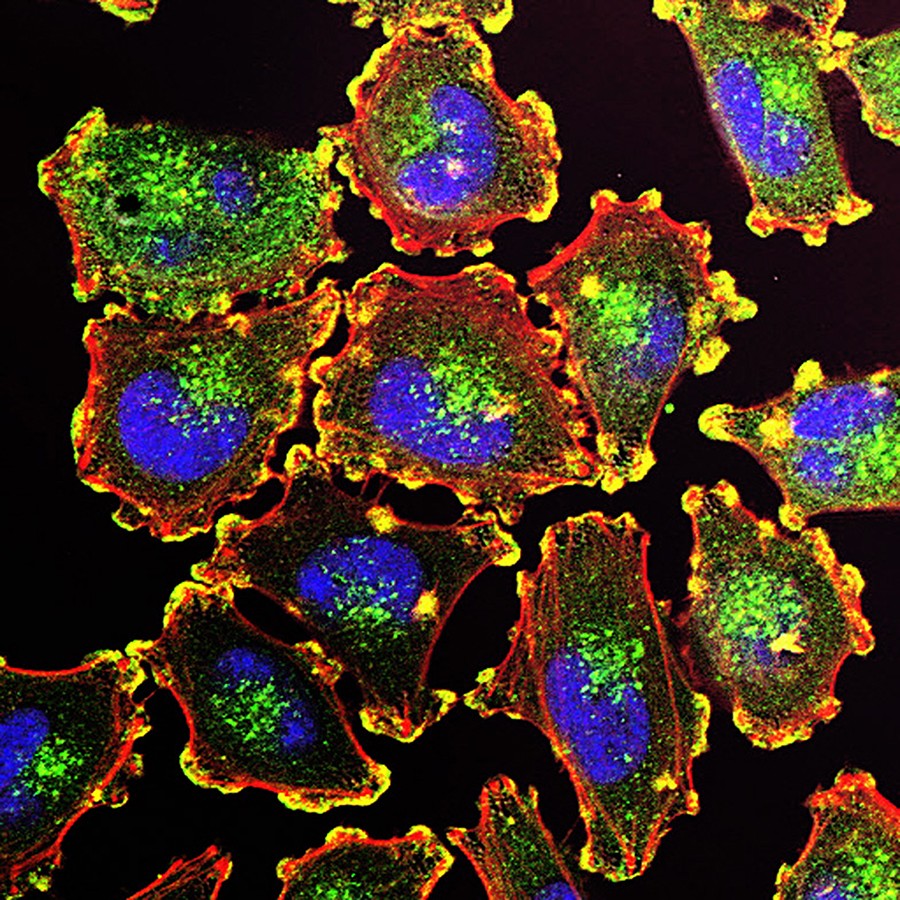
By studying 151 samples of both melanoma tumors and normal skin, scientists created a detailed map of proteins within each cell. What they found was startling. The most aggressive melanomas weren’t just growing fast—they were turbocharging the cellular machinery that builds mitochondrial proteins and ramping up how mitochondria convert nutrients into usable energy.
This wasn’t a coincidence. It was a tactic. Cancer cells, in their hunger to divide and survive, had rewired their inner engines.
But that dependency may now be melanoma’s Achilles’ heel.
Old Drugs, New Purpose
With that vulnerability exposed, the research team turned to existing drugs to see what would happen if they shut the power off. They used two types: one, antibiotics originally designed to block bacteria from making proteins; the other, drugs that inhibit mitochondrial energy production.
Because mitochondrial protein synthesis shares ancestry with bacterial systems (a clue from evolution), these antibiotics were surprisingly effective at disrupting the cancer’s power supply.
The result? Melanoma cells collapsed. In laboratory dishes, the cancer could not survive. Meanwhile, non-cancerous skin cells were left largely unharmed—a crucial signal that the treatment is not only powerful, but precise.
“This discovery identifies melanoma’s excessive reliance on mitochondrial energy as its Achilles’ heel,” said senior author Dr. Jeovanis Gil of Lund University. “It reveals a therapeutic vulnerability that we can exploit with existing drugs.”
It’s a powerful idea—using the very machinery cancer needs for survival as the target for its destruction.
Precision Medicine Finds a New Target
But the implications stretch beyond just a single study or type of drug. Because the mitochondrial overdrive is traceable—it leaves behind a measurable protein fingerprint—doctors may soon be able to identify which patients have this specific vulnerability.
That opens the door to personalized therapy.
The mitochondrial-protein signature, detectable in standard tumor biopsies, could become a biomarker. Patients with tumors that show this signature might benefit most from these mitochondrial-targeted therapies. Rather than a one-size-fits-all approach, treatment could be tailored to each tumor’s biology.
That’s the promise of precision medicine: understanding not just where the cancer is, but how it behaves at the cellular level—and then choosing the perfect treatment match.
“By pairing mitochondrial blockers with today’s standards of care,” Dr. Gil added, “we may cut off a major escape route that cancers use to resist therapy and come back.”
A Doorway to Broader Cancer Therapies
This research isn’t just about melanoma. Mitochondrial rewiring—this subtle cellular sabotage—has been observed across multiple cancers. Tumors of the lung, pancreas, and brain have all shown signs of mitochondrial dependency, especially when under pressure from treatment.
By proving that mitochondrial-targeted therapies can cripple melanoma, the Lund team may have opened a new frontier in cancer care.
Imagine combining these old-but-rediscovered drugs with immunotherapy, radiation, or chemotherapy. Imagine turning a corner in cancers that have long resisted treatment. The key, it turns out, might have been hiding in the cell’s ancient engines all along.
What Comes Next
Of course, lab dishes are not living bodies, and promising results in the lab must undergo clinical trials before becoming standard care. But the elegance of this study—targeting a process cancer cells depend on more than normal cells—offers a high signal of hope.
And with the tools already available, clinical testing could begin sooner rather than later.
For now, researchers are working to refine which drug combinations are most effective and safest, and how to integrate mitochondrial inhibitors with current therapies.
But something has changed. For a cancer long known for its cunning resistance, researchers now have a new target. Not the surface of the cell. Not even its genes. But its power core.
And in a twist both poetic and powerful, the very engine melanoma cells need to live may soon become the mechanism of their fall.
Reference: Mitochondrial Proteome Landscape Unveils Key Insights into Melanoma Severity and Treatment Strategies, Cancer (2025). DOI: 10.1002/cncr.35897