Ear infections have long been a rite of passage for infants and toddlers. Red cheeks, high fevers, sleepless nights, and endless tears—these are the hallmarks familiar to millions of parents watching their child suffer through a middle ear infection, known medically as otitis media. Doctors usually respond with a week’s course of oral antibiotics. While effective, this approach is far from perfect. Side effects, from upset stomachs to yeast infections, often complicate treatment, leading to missed doses, lingering infections, and, increasingly, antibiotic resistance.
But what if all it took was one dose? No swallowing. No repeated syringes. Just a single, topical application.
Thanks to a revolutionary study published in ACS Nano, that vision is now closer to reality. A team of researchers has developed a temperature-sensitive antibiotic gel that—when applied once to the outer ear—eradicated middle ear infections in chinchillas within 24 hours. More than a novel formulation, it’s a potential game-changer for pediatric care.
The Painful Plague of Ear Infections
Middle ear infections are one of the most common reasons young children visit the doctor and are prescribed antibiotics. These infections occur behind the eardrum, in a small, air-filled chamber known as the middle ear. When bacteria or viruses invade this space—often following a cold or respiratory infection—fluids build up, pressure increases, and pain intensifies.
While oral antibiotics like amoxicillin or ciprofloxacin are usually prescribed, these drugs circulate throughout the entire body, even though the infection is confined to a tiny, inaccessible space. The side effects can be substantial: gastrointestinal distress, allergic reactions, and disruptions to a child’s natural microbiome. Moreover, finishing a full course of oral antibiotics is often a challenge, especially in fussy or unwell children.
This inefficiency in treatment not only frustrates parents and caregivers—it also fuels one of the 21st century’s most pressing health issues: antibiotic resistance. The more often antibiotics are used (especially improperly or unnecessarily), the more likely bacteria will evolve resistance to them.
Enter Dr. Rong Yang and her colleagues at Cornell University, who have reimagined how we treat these infections—by targeting them directly at the source.
Rethinking Treatment: From Pills to Precision
Topical treatments—those applied directly to the affected area—are common in dermatology, dentistry, and ophthalmology. But for ear infections, this approach has been largely impractical. The eardrum, or tympanic membrane, separates the outer ear from the middle ear and acts as a nearly impenetrable barrier to most substances, including medications.
But what if scientists could persuade an antibiotic to slip through?
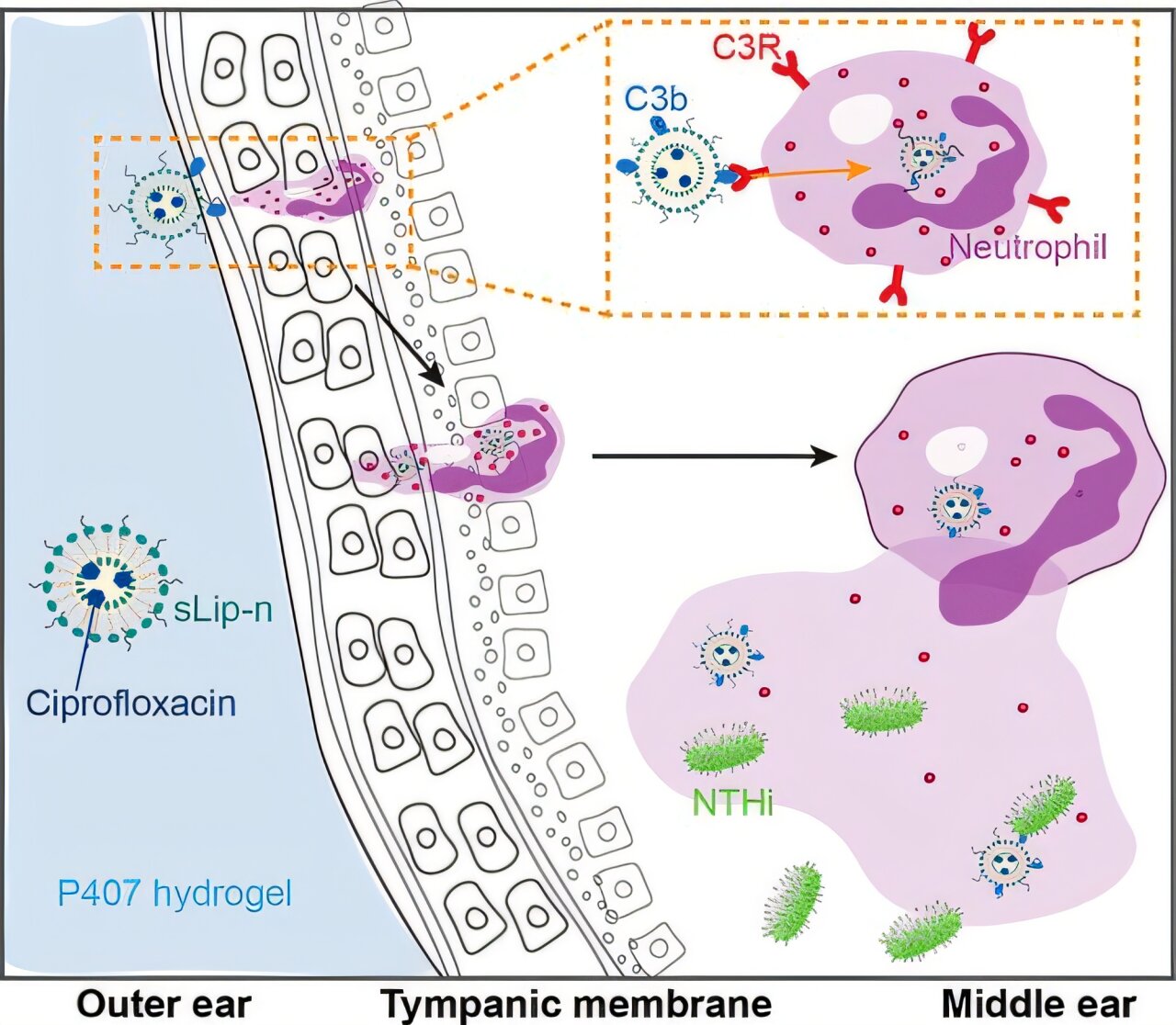
Dr. Yang and her collaborator Dr. Wenjing Tang set out to solve this longstanding problem. Their strategy hinged on a clever form of molecular disguise: using liposomes—tiny, spherical packets made from fat-like substances—to smuggle antibiotics through the eardrum. These liposomes can merge with or be absorbed by biological membranes, and they’ve already proven successful in delivering cancer drugs and vaccines.
Here, however, the challenge wasn’t just getting through a healthy eardrum—it was breaching an infected one.
The Curious Case of the Negative Charge
Liposomes come in different varieties. Some carry a positive electrical charge, others a negative one. Conventional wisdom suggested that positively charged liposomes would be superior for drug delivery, particularly across multilayered biological tissues like skin.
Yet, when Yang and her team tested both forms, they discovered something surprising: the negatively charged liposomes performed better in delivering antibiotics across infected eardrums.
Why? The answer lies in the immune system. Infected tissue recruits immune cells—natural first responders that engulf pathogens and foreign particles. The researchers found that these immune cells preferentially absorbed the negatively charged liposomes, acting as inadvertent delivery agents for the antibiotic payload. Once inside the immune cells, the liposomes released the antibiotic ciprofloxacin, which then penetrated deeper into the middle ear.
It was a bit like sneaking medicine into a fortress by convincing the guards to carry it inside.
A Gel That Changes with Temperature
To enhance the liposomes’ effectiveness and make them easy to apply, the team embedded them in a hydrogel—a jelly-like material that changes consistency with temperature. At room temperature, the gel remains liquid and easy to apply. But once it reaches body temperature, it solidifies into a semi-firm matrix, slowly releasing the drug over time.
This temperature-sensitive behavior ensures that the antibiotic stays in place, rather than dripping out or being dislodged. It also means a single application could provide sustained treatment, eliminating the need for multiple doses.
To test the approach, the researchers turned to chinchillas—furry, wide-eyed rodents whose ear anatomy closely resembles that of humans. In their experiments, the researchers infected chinchillas’ middle ears and treated them with one of three formulations: ciprofloxacin in a plain gel, ciprofloxacin in positively charged liposomes within gel, and ciprofloxacin in negatively charged liposomes within gel.
A Stunning Cure in 24 Hours
The results were stunning.
All the animals treated with the negatively charged liposome gel were completely infection-free within 24 hours. There was no recurrence of infection and no inflammation observed for an entire week. In contrast, only 50% of the chinchillas receiving the positively charged liposome gel were cured, and just 25% of those who received plain ciprofloxacin gel showed improvement. These animals still exhibited signs of inflammation—suggesting that a one-dose, non-targeted treatment was insufficient.
What makes this so promising isn’t just the speed of the cure—but the precision. The gel delivers the antibiotic exactly where it’s needed, minimizing systemic exposure and reducing the risk of side effects and resistance.
Dr. Yang expressed her excitement: “I often receive emails from parents asking when our formulation will be available, and I share their hope for a solution. A single-dose treatment for middle ear infections represents a significant step forward toward reducing the burden on families and improving outcomes for young children.”
From Lab Bench to Medicine Cabinet
While these results are preliminary and limited to animal models, the implications are massive. Translating the technology from chinchillas to children will involve rigorous clinical trials to assess safety, efficacy, and tolerability. But the foundational science is strong—and the need is urgent.
The antibiotic gel could dramatically reduce the amount of pediatric antibiotic use, improving compliance (especially in younger children who struggle with swallowing medication) and reducing the pressure that drives bacterial resistance. It could also help reduce healthcare costs by limiting repeat visits, additional prescriptions, and complications from untreated infections.
“I am most excited about the next stage of translating this technology from the lab to the clinic,” said Yang. “It has the potential to improve patient compliance, reduce antibiotic resistance, and ultimately transform how children receive antibiotics.”
Rewriting the Rules of Pediatric Care
If this gel eventually reaches pharmacies, it could represent one of the most significant advances in pediatric infectious disease treatment in decades. Imagine a routine doctor’s visit ending not with a 10-day prescription and worried instructions, but with a single, in-office application and peace of mind.
Ear infections, for all their ubiquity, are serious. Repeated infections can lead to hearing loss, speech delays, and poor academic performance. For many families, they’re also a source of financial strain and emotional exhaustion. A one-and-done treatment could offer tremendous relief.
And beyond the ear? This technology opens the door to new treatments for other infections tucked behind difficult barriers: sinus infections, dental abscesses, and even lung infections, where direct delivery could bypass systemic risks. The use of smart hydrogels and targeted liposomes may become a broader paradigm in antibiotic therapy.
A Future Without Overused Antibiotics?
As antibiotic resistance continues to rise, researchers around the world are exploring alternatives: bacteriophage therapy, antimicrobial peptides, and drug-free immunomodulation. But perhaps the most immediate step forward lies in using our existing tools more intelligently.
By delivering antibiotics precisely where they’re needed—in small, effective doses—we can reduce the overuse that drives resistance. This gel is not just a scientific innovation. It’s a statement: that we can do better for our children, for our bacteria-fighting arsenal, and for our future.
In a world where infections are growing stronger, smart delivery systems may be our strongest defense. And with each advance like this, we get a little closer to a time when antibiotics are used not just more sparingly—but more wisely.
Reference: Wenjing Tang et al, Pathophysiology-Informed Design of Negatively Charged Liposomes for Enhanced Antibiotic Delivery across the Intact Tympanic Membrane in Acute Otitis Media Treatment, ACS Nano (2025). DOI: 10.1021/acsnano.4c14097