Imagine if humans could capture sunlight as efficiently as plants do and turn it into clean, carbon-neutral fuel. A team of scientists at the University of Basel, Switzerland, has taken a remarkable step toward this vision. Their research focuses on mimicking the natural process of photosynthesis—the method by which plants convert sunlight and carbon dioxide into energy-rich molecules—to create sustainable solar fuels for the future.
The Power of Photosynthesis
Photosynthesis is one of nature’s most elegant processes. Plants absorb sunlight to convert carbon dioxide and water into carbohydrates, storing energy in chemical bonds. Animals, including humans, then consume these carbohydrates and “burn” them for energy, releasing carbon dioxide back into the environment. This cycle, simple yet profound, sustains nearly all life on Earth.
Scientists have long dreamt of replicating this process artificially, using sunlight to generate energy-rich compounds like hydrogen, methanol, or synthetic gasoline. The ultimate goal is to create fuels that are carbon-neutral: burning them releases only the same amount of carbon dioxide that was absorbed during their production. Achieving this could transform our approach to energy, reducing reliance on fossil fuels and combating climate change.
A Molecule Designed for Light
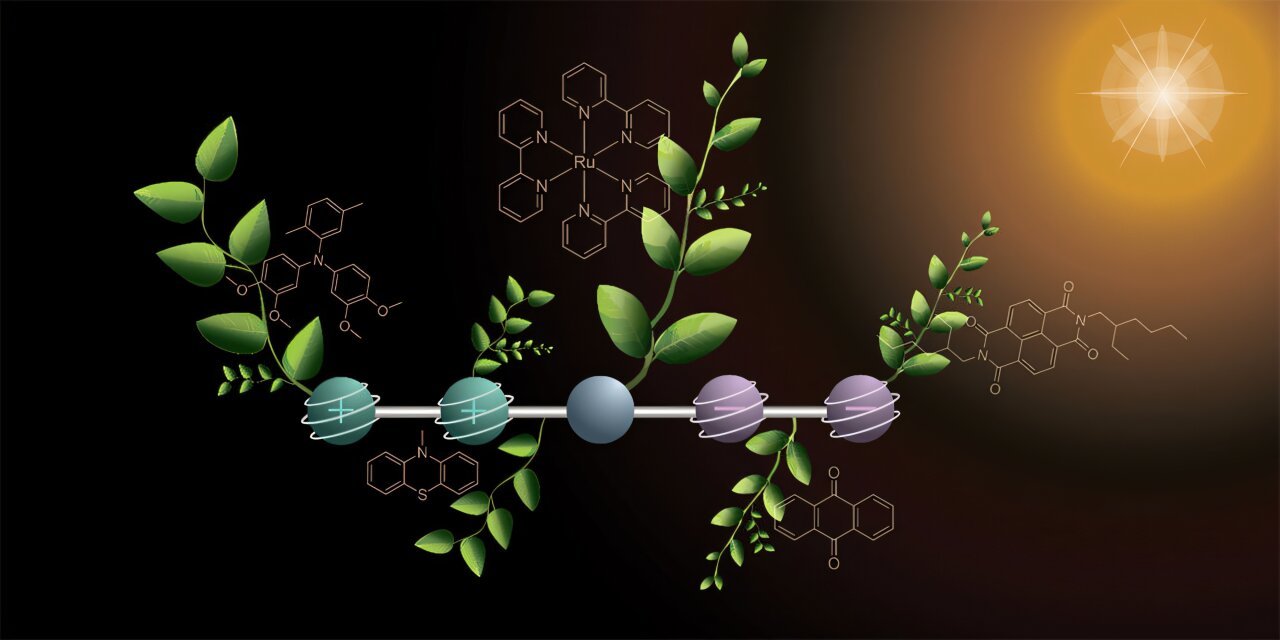
At the heart of this research is a specially designed molecule capable of storing multiple electric charges at once. Professor Oliver Wenger and his doctoral student Mathis Brändlin reported their findings in Nature Chemistry, describing a molecule that can hold two positive and two negative charges simultaneously when exposed to light.
Why is this significant? Storing multiple charges is crucial for converting sunlight into chemical energy. These charges can drive essential reactions, such as splitting water into hydrogen and oxygen—an important step toward producing clean fuels.
The molecule itself is a marvel of engineering. It consists of five linked components, each with a precise role. On one side, two segments release electrons and acquire positive charges. On the other side, two segments absorb these electrons, becoming negatively charged. Sandwiched between them is a component that captures sunlight and initiates the electron transfer. Together, these parts create a system capable of controlled energy storage and movement, mimicking the electron flow in natural photosynthesis.
Generating Charges, Step by Step
The researchers employed a clever, two-step approach to generate four charges. The first flash of light triggers a reaction that produces one positive and one negative charge, which then travel to opposite ends of the molecule. A second flash repeats the process, resulting in two positive and two negative charges stored simultaneously.
This stepwise excitation allows the molecule to work even under dim light conditions, approaching the intensity of natural sunlight. Previous experiments required powerful laser light, making practical applications unrealistic. Now, the charges remain stable long enough to be harnessed for subsequent chemical reactions—a vital step toward building a fully functional artificial photosynthesis system.
A Key Piece of the Puzzle
While this molecule does not yet create a complete artificial photosynthesis system, it represents a critical milestone. Wenger emphasizes that their work sheds light on the complex electron transfers at the core of artificial photosynthesis. Understanding and controlling these transfers is essential for turning sunlight into usable fuel efficiently.
“This stepwise excitation brings us closer to sunlight-level intensities,” Brändlin explains. “We are not just generating charges; we are learning how to store and use them in ways that could ultimately lead to sustainable energy production.”
Toward a Sustainable Energy Future
The implications of this research are profound. Artificial photosynthesis has the potential to provide a renewable, carbon-neutral source of fuel, offering a solution to the dual crises of climate change and energy scarcity. By learning from nature and refining molecular design, scientists are laying the groundwork for energy technologies that could power homes, vehicles, and industries without adding to atmospheric carbon.
Wenger concludes with optimism: “We hope that this research opens new prospects for a sustainable energy future. Every discovery, every molecule, brings us a step closer to a world powered by sunlight rather than fossil fuels.”
A Vision Within Reach
The journey from understanding a molecule to powering a carbon-neutral world is long, but this research shows that each step matters. By capturing and storing energy like a leaf, humans may one day emulate one of nature’s most fundamental processes. The dream of turning sunlight into fuel, once the realm of science fiction, is gradually becoming reality, guided by ingenuity, persistence, and a deep respect for the natural world.
Artificial photosynthesis, like the molecule developed by Wenger and Brändlin, reminds us that the solutions to our most pressing challenges may lie in the elegant simplicity of nature, waiting for us to understand, imitate, and improve.
More information: Photoinduced Double Charge Accumulation in a Molecular Compound, Nature Chemistry (2025). DOI: 10.1038/s41557-025-01912-x