Sleep may seem like a passive, unproductive state, but it is one of the most deeply conserved biological behaviors across species—from humans to birds to insects. Even the humble fruit fly, Drosophila melanogaster, dozes off in regular cycles, a puzzling fact given its minuscule brain and straightforward lifestyle. Why, then, do organisms need to sleep? What internal signals tell a brain—regardless of species—that it’s time to rest?
A groundbreaking new study from the Max Planck Institute for Neurobiology of Behavior–Caesar (MPINB) in Germany provides fascinating clues to this mystery. Published in Nature Neuroscience, the research highlights a role long overlooked in sleep biology: glial cells—the brain’s non-neuronal but vital support staff—are acting as internal metabolic monitors, subtly steering sleep, feeding, and rest in fruit flies. This isn’t just a study about insect biology. It’s a striking insight into the ancient, cellular logic that governs homeostasis—and possibly your next yawn.
Beyond Neurons: The Unsung Architects of Homeostasis
Homeostasis is the fundamental biological process by which living organisms maintain internal stability despite an ever-changing external world. Whether it’s body temperature, hydration, pH, or blood sugar, maintaining balance is essential to survival. But beyond the better-known physiological regulators, there’s a behavioral dimension to homeostasis: sleep, feeding, rest. These behaviors don’t just reflect needs—they actively restore equilibrium.
Historically, much of the scientific spotlight has focused on neurons, the electrically excitable messengers of the nervous system. Yet neurons don’t act alone. Glial cells—once dismissed as mere ‘brain glue’—have emerged as crucial regulators of the nervous system’s most complex and mysterious tasks. They insulate axons, clear debris, modulate neurotransmission, and—according to this new research—may even determine when we sleep and when we eat.
Why Fruit Flies? The Power of Simple Brains
Dr. Andres Flores-Valle, first author of the MPINB study, sees Drosophila melanogaster not as a simplified organism, but as an elegant model for decoding the primal laws of life. “Humans spend about a third of their lives sleeping, yet we still don’t fully understand why sleep is necessary,” he explains. “Our research aims to answer this question by studying fruit flies, which despite having much simpler brains, also sleep. Their simplicity and the powerful genetic tools available make them ideal for understanding the basic functions of sleep.”
Fruit flies may not dream or snore, but their sleep exhibits many of the hallmarks seen in humans: cycles of activity and rest, rebound after deprivation, and even preference for darkness. More importantly, their tiny brains can be imaged in real time, allowing scientists to observe, cell by cell, how sleep unfolds.
Comfortable Flies, Clever Tech: A Window Into the Sleeping Brain
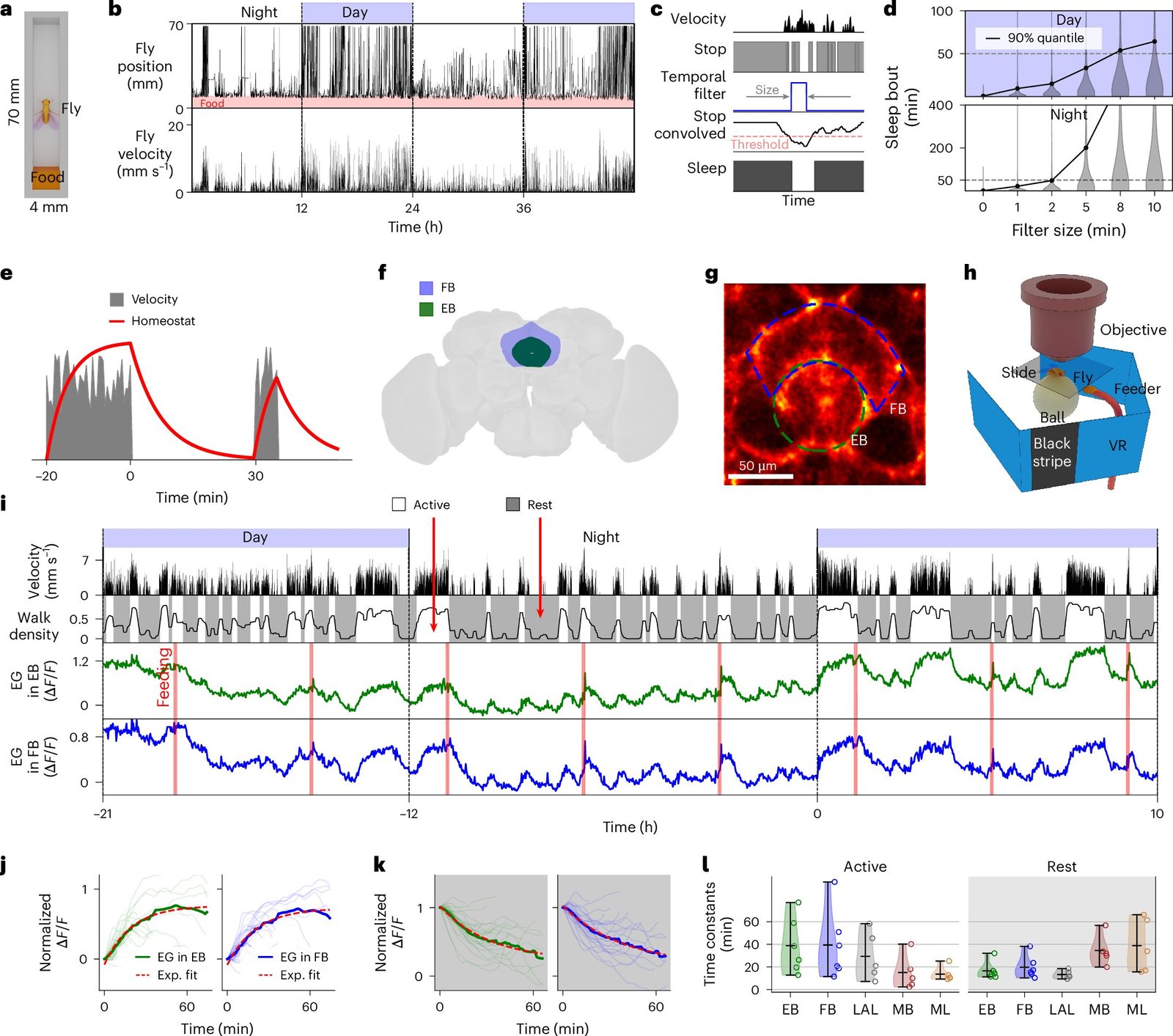
To unravel the mystery of sleep regulation, Flores-Valle and his team took an unusual approach: they made flies comfortable. Instead of shocking or restraining them, they gently fed the flies every few hours and allowed them to sleep naturally under a microscope. A specialized robotic system ensured that their needs were met, while high-resolution in vivo imaging recorded the activity of glial and neural cells over multiple days.
This innovative setup allowed the team to observe transitions between sleep and wakefulness in real time, a major technical leap. While many previous studies relied on snapshots or invasive probes, this continuous monitoring offered a living, breathing map of how the fly brain tracks internal states.
Two Types of Glia, Two Behavioral Gateways
The researchers focused on two glial cell types: astrocyte-like glia and ensheathing glia. Astrocyte-like glia are comparable to mammalian astrocytes, cells that help maintain chemical equilibrium around neurons and regulate synaptic activity. Ensheathing glia, meanwhile, wrap around axons and aid in structural repair, metabolic exchange, and cellular cleanup.
Their role, it turns out, is far more dynamic than once thought. Using calcium imaging—a technique that tracks intracellular calcium levels as a proxy for activity—the researchers discovered that both glial types show rhythmic activity changes linked to the fly’s behavioral state.
Calcium levels in glial cells rose progressively during periods of wakefulness and then dropped after sleep. In other words, these cells tracked sleep need. As the fly stayed awake longer, the calcium signal increased, almost as if the glia were tallying fatigue. Once the fly slept, the signal reset.
But here’s where things get even more intriguing: neurons, long thought to be the primary drivers of sleep, were instead found to be more responsive to feeding. Their activity rose steadily until the fly ate, then declined. This startling observation flips a long-held assumption: glia, not neurons, may be the true gatekeepers of sleep need.
The Metabolic Connection: Clearing CO₂, Balancing pH, Restoring Equilibrium
What drives this glial calcium activity? The researchers believe the answer lies in metabolism. During wakefulness, cellular processes ramp up, leading to accumulation of metabolic byproducts like carbon dioxide and shifts in pH levels. Glial cells respond to these changes, acting as metabolic sentinels. The rising calcium levels appear to reflect the growing urgency to restore balance—a signal that it’s time to rest and reset.
In essence, sleep may serve as a nightly detox, during which glia recalibrate the brain’s chemical environment. This offers a deeply integrative view of sleep: not just as a neural phenomenon, but as a metabolic necessity.
“We found that glial calcium activity is linked to metabolism—helping clear CO₂ and regulate pH—suggesting sleep may be needed to restore metabolic balance,” said Flores-Valle.
This insight resonates with human sleep research as well. Studies have shown that brain metabolism changes dramatically during sleep. In deep sleep, the brain’s waste clearance system—known as the glymphatic system—kicks into high gear. Disruptions in this process have been linked to neurological diseases, including Alzheimer’s. The parallels with fruit fly glia are striking and suggest a deep evolutionary conservation of these mechanisms.
Sleep, Feeding, and the Brain’s Unified Economy
The separation of tasks between glia and neurons also hints at a more nuanced view of brain function. Rather than a single, monolithic control center, the brain may operate more like a dynamic marketplace, with different cell types specializing in different forms of currency: glia tracking metabolic debt, neurons responding to caloric intake.
This division of labor could be crucial for efficiency. A system that tracks both feeding and sleep independently allows organisms to adapt flexibly to changing environments—sometimes skipping rest to find food, or delaying a meal to ensure recuperation.
Moreover, this discovery may help explain why sleep and metabolism are so deeply intertwined. Sleep disorders and metabolic diseases often go hand in hand, from obesity to diabetes to depression. Understanding the cellular dialogue between glia and neurons could provide new avenues for treating these conditions.
What Comes Next: A Search for the Sleep Switch
While this study illuminated how glial activity correlates with sleep need, it raised an even deeper question: how exactly does elevated calcium in glia trigger the brain to sleep?
Flores-Valle and his colleagues suspect that glia don’t merely observe the buildup of metabolic stress—they may initiate the very signals that silence the brain. In their next phase of research, they aim to map how glial signals propagate through neural circuits, leading to the behavioral switch from wakefulness to rest.
“Our next studies will try to uncover how glial cells trigger the transition from wakefulness to sleep, a key question that remains unanswered,” he said. “We suspect that elevated calcium activity in glia after prolonged wakefulness helps initiate sleep, but how the signal acts across the brain is still unknown.”
From Fruit Flies to Humans: A Universal Sleep Code?
The implications of this research stretch far beyond insect biology. If glial cells play a similar role in humans, they may be a missing link in our understanding of sleep disorders, fatigue syndromes, and even neurodegeneration. Already, evidence from rodent models suggests that astrocytes help regulate sleep pressure. Now, with fruit flies providing a genetically precise platform, the field is poised to unlock the basic rules governing sleep across species.
This work is also a reminder of science’s recursive nature. Sometimes, to answer the grandest questions—Why do we sleep? How does the brain know it’s tired?—we must zoom into the tiniest creatures, where the answers are written not in words, but in waves of calcium flickering through glial cells.
In the end, sleep may not be a passive surrender, but a proactive, metabolically driven act—engineered by silent glial sentinels that balance the brain’s invisible equations. Thanks to a team of fly-whisperers in Germany, we’re a step closer to understanding how that nightly mystery unfolds, and why it matters more than we ever imagined.
Reference: Andres Flores-Valle et al, Dynamics of glia and neurons regulate homeostatic rest, sleep and feeding behavior in Drosophila, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-01942-1