In the long history of science, certain discoveries arrive quietly, almost modestly, before revealing their true magnitude. Graphene is one of those discoveries. It is a material so thin it is effectively two-dimensional, so strong it defies everyday intuition, and so versatile it seems to blur the boundary between science fiction and engineering reality. For a time, it was spoken of in almost mythical terms, a “wonder material” that would revolutionize electronics, energy, medicine, and materials science all at once. Yet graphene is more than hype, more than headlines and promises. It is a profound scientific achievement that reshaped how we think about matter itself.
Graphene’s story is not just about a new substance. It is about curiosity, patience, and the slow, often frustrating journey from discovery to application. It is about how nature, when examined closely enough, can still surprise us. To understand why graphene inspired such excitement, and why its revolution has unfolded more slowly than expected, we must look closely at what graphene is, how it behaves, and what it taught us about the limits and possibilities of the material world.
A Single Layer That Changed Materials Science
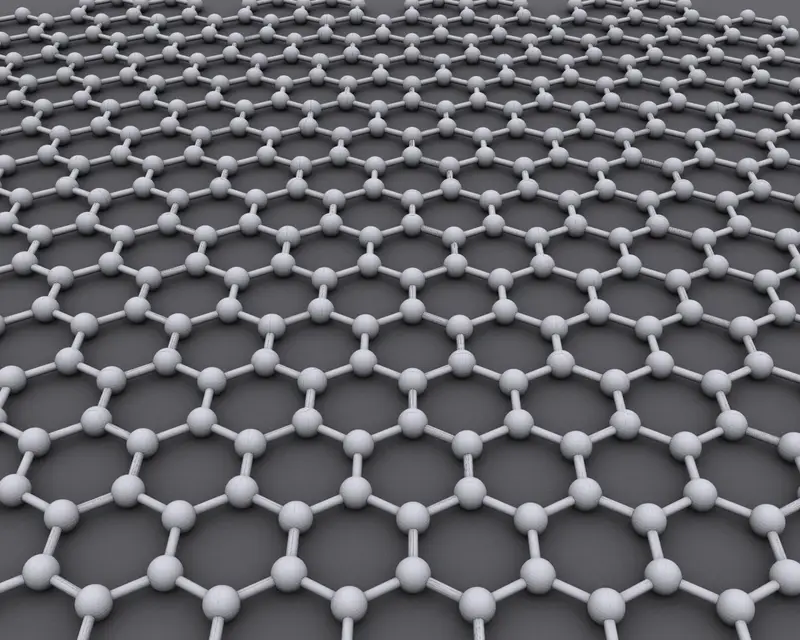
At its simplest, graphene is a single layer of carbon atoms arranged in a hexagonal, honeycomb-like pattern. Carbon atoms bond to three neighbors, forming a flat sheet only one atom thick. This description sounds almost trivial, but its implications are extraordinary. For decades, physicists believed that such a perfect two-dimensional crystal could not exist in reality. According to theoretical arguments, thermal vibrations should tear it apart. Graphene was expected to be unstable, a mathematical curiosity rather than a physical object.
Yet nature had other plans. When graphene was finally isolated, it proved not only stable but astonishingly robust. This single atomic layer exhibited properties unlike anything seen before. It was stronger than steel by weight, more conductive than copper, and nearly transparent while still being an excellent conductor of electricity. Suddenly, the idea of two-dimensional materials moved from theory into tangible reality.
The discovery forced scientists to rethink long-held assumptions. Graphene showed that the rules governing matter can change dramatically when dimensions are reduced. In one atom-thick materials, electrons behave differently, vibrations take on new forms, and strength emerges from geometry rather than bulk. Graphene was not just a new material; it was a new way of looking at matter.
The Accidental Discovery That Wasn’t Supposed to Work
Graphene’s isolation is often described as almost playful in its simplicity. Researchers used ordinary adhesive tape to peel layers from graphite, the same material found in pencil lead. By repeatedly sticking and peeling, they thinned the graphite down to individual layers. Against expectations, some of these layers turned out to be single sheets of carbon atoms.
What made this discovery remarkable was not just the technique but the mindset behind it. The experiment worked because the researchers were willing to try something that seemed unlikely to succeed. They did not set out with a grand industrial process or expensive equipment. Instead, they followed curiosity and intuition, allowing room for surprise.
This moment highlighted an important truth about science: breakthroughs often emerge not from rigid plans but from openness to the unexpected. Graphene’s discovery was a reminder that even in a world of advanced instruments and complex theories, simple experiments can still unlock profound insights.
Carbon’s Remarkable Versatility
Carbon is already famous for its versatility. It forms the backbone of life, appears in materials as soft as graphite and as hard as diamond, and creates complex molecular structures unmatched by any other element. Graphene is another chapter in this story, revealing yet another face of carbon’s adaptability.
What makes graphene unique among carbon materials is its perfect two-dimensionality. In graphite, many graphene layers stack on top of each other, interacting weakly. In graphene itself, those interactions disappear, allowing the intrinsic properties of a single layer to emerge. Electrons move through this flat lattice as if they have no mass, behaving according to rules more commonly associated with high-energy physics than with everyday materials.
This behavior is not a mere curiosity. It explains graphene’s exceptional electrical conductivity and its sensitivity to external influences. Tiny changes in environment, such as the presence of a single molecule, can noticeably affect graphene’s electrical properties. This sensitivity makes graphene attractive for sensors, while its robustness makes it appealing for structural applications.
Strength Beyond Intuition
One of graphene’s most celebrated properties is its strength. Despite being only one atom thick, graphene is extraordinarily strong and stiff. Stretching it requires immense force relative to its weight. This strength arises from the carbon-carbon bonds within the hexagonal lattice, which are among the strongest chemical bonds known.
What makes this strength emotionally striking is the contrast between appearance and reality. Graphene is nearly invisible, so thin that it seems fragile by definition. Yet at the atomic level, it is a tightly bound network that resists deformation. This paradox challenges our everyday understanding of materials, where thickness and strength usually go hand in hand.
The idea that something so thin can be so strong has inspired visions of ultralight, ultra-strong materials. From protective coatings to advanced composites, graphene has been imagined as a way to make structures stronger without adding weight. While translating this promise into real-world products has proven difficult, the fundamental insight remains powerful: strength can emerge from structure, not just mass.
Electrical Conductivity and the Dream of Faster Electronics
Graphene’s electrical properties fueled much of the early excitement surrounding it. Electrons in graphene move with extraordinary mobility, encountering little resistance as they travel across the lattice. This means that graphene can carry electrical current extremely efficiently, even at room temperature.
In theory, this made graphene an ideal candidate for next-generation electronics. Faster transistors, flexible displays, transparent conductors, and low-power devices all seemed within reach. The image of rollable screens and ultrafast processors captured public imagination.
Yet here, the story becomes more complex. Graphene lacks a natural band gap, a feature essential for conventional digital electronics. Without a band gap, it is difficult to switch current on and off completely, a requirement for logic circuits. Researchers developed clever ways to modify graphene or combine it with other materials, but these solutions often compromised its remarkable conductivity.
This tension between ideal properties and practical requirements illustrates a recurring theme in materials science. A material can be extraordinary in isolation but challenging to integrate into existing technologies. Graphene did not replace silicon overnight, but it deepened our understanding of electronic materials and inspired new approaches to device design.
Transparency and Flexibility
Another striking feature of graphene is its transparency. A single layer absorbs only a small fraction of visible light, making it almost perfectly transparent. At the same time, it remains electrically conductive and mechanically flexible.
This combination is rare and valuable. Traditional transparent conductors rely on materials that are brittle or require scarce elements. Graphene offered a vision of transparent electrodes that could bend, stretch, and endure repeated use. Touchscreens, solar cells, and wearable electronics all seemed poised for transformation.
In practice, producing large-area, defect-free graphene sheets proved to be a major challenge. Imperfections disrupt electrical pathways and weaken mechanical performance. Manufacturing methods improved steadily, but scaling up while maintaining quality remained difficult and expensive. Still, the pursuit of graphene-based transparent conductors drove innovation in fabrication techniques and broadened the field of flexible electronics.
Thermal Conductivity and Heat Management
Graphene is not only an excellent conductor of electricity; it is also an exceptional conductor of heat. Heat travels through graphene with remarkable efficiency, making it one of the best thermal conductors known.
This property opened up possibilities in heat management, an increasingly critical issue in modern technology. As electronic devices become smaller and more powerful, removing heat efficiently becomes essential to prevent damage and maintain performance. Graphene’s ability to spread heat rapidly suggested it could serve as an effective thermal interface material.
The challenge, once again, lay in integration. Transferring graphene’s intrinsic thermal properties to macroscopic systems requires careful control over interfaces and bonding. Even so, research into graphene’s thermal behavior enriched our understanding of heat transport at the nanoscale and influenced the design of new materials inspired by similar principles.
Graphene and the Birth of Two-Dimensional Materials
Perhaps graphene’s most enduring legacy is the door it opened to an entirely new class of materials. Once scientists demonstrated that a single atomic layer could exist and be stable, attention turned to other layered materials. Soon, a family of two-dimensional materials emerged, each with its own unique properties.
Some exhibited natural band gaps, making them suitable for electronics. Others showed strong interactions with light or unusual magnetic behavior. Graphene became the prototype, the reference point against which all other two-dimensional materials were compared.
This expansion transformed condensed matter physics and materials science. Researchers began stacking different two-dimensional layers, creating artificial materials with tailored properties. These layered structures revealed new phenomena, including exotic electronic states and tunable optical responses. In this sense, graphene’s true impact may lie not in its direct applications but in the conceptual revolution it sparked.
Chemical Sensitivity and Sensing Applications
Graphene’s surface is entirely exposed, with every atom available to interact with the environment. This makes it extraordinarily sensitive to chemical changes. Even the adsorption of a single molecule can alter its electrical properties.
This sensitivity suggested applications in sensing, from detecting gases to monitoring biological molecules. Graphene-based sensors promised high sensitivity, fast response, and low power consumption. In controlled laboratory settings, these sensors demonstrated impressive performance.
Translating this sensitivity into reliable, selective sensors for real-world use proved challenging. Distinguishing between different molecules, maintaining stability over time, and protecting against environmental interference required sophisticated designs. Nevertheless, graphene’s role in advancing sensor technology is undeniable, influencing approaches to detection and measurement across multiple fields.
Graphene in Energy Storage and Conversion
Energy technologies were another area where graphene inspired hope. Its high surface area, conductivity, and mechanical stability made it attractive for batteries, supercapacitors, and fuel cells. Graphene was envisioned as a way to store more energy, charge faster, and extend device lifetimes.
In practice, graphene often served best as a component rather than a standalone solution. When combined with other materials, it improved conductivity, structural integrity, or durability. These incremental improvements might seem modest compared to early hype, but they reflect a more realistic understanding of how materials contribute to complex systems.
The story of graphene in energy technology underscores an important lesson. Revolutionary materials rarely act alone. Their greatest value often emerges when they complement and enhance existing technologies rather than replace them outright.
The Gap Between Promise and Reality
At the height of excitement, graphene was described as a material that would change everything. When such sweeping transformations did not immediately materialize, disappointment followed. Some critics dismissed graphene as overhyped, pointing to the slow pace of commercialization.
Yet this perspective overlooks the natural timescale of materials innovation. From discovery to widespread application, decades often pass. Silicon itself took many years to move from laboratory curiosity to the foundation of modern electronics. Graphene’s journey is still unfolding.
The gap between promise and reality reflects not failure but complexity. Integrating a new material into industrial processes requires overcoming technical, economic, and logistical barriers. Graphene challenged existing manufacturing paradigms, demanding new approaches to production, quality control, and design.
Manufacturing Challenges and Progress
Producing graphene in large quantities while preserving its exceptional properties remains one of the central challenges. Different methods yield graphene with different characteristics, suitable for different applications. Mechanical exfoliation produces high-quality graphene but is not scalable. Chemical methods offer scalability but introduce defects.
Balancing quality, cost, and scalability is an ongoing effort. Significant progress has been made, and graphene is now produced commercially for various uses. While these forms may not match the perfection of laboratory samples, they are good enough to deliver meaningful improvements in real-world products.
This evolution highlights the difference between idealized materials and practical ones. Real materials always involve compromises, and engineering is the art of choosing which compromises matter most.
Graphene’s Influence on Science and Culture
Beyond its technical impact, graphene captured the imagination of scientists and the public alike. It became a symbol of modern materials science, representing the idea that revolutionary discoveries can still be made with simple tools and bold thinking.
Graphene also influenced how science is communicated. It showed how quickly a discovery can move from academic journals to popular media, and how narratives of transformation can shape expectations. The experience prompted reflection within the scientific community about hype, responsibility, and the communication of uncertainty.
In this way, graphene played a role not only in advancing knowledge but in shaping the relationship between science and society.
The Quiet Revolution Still Underway
Today, graphene is no longer a novelty. It is a mature field of research, integrated into a broader landscape of advanced materials. Its most dramatic promises have softened into more measured expectations, but its influence continues to grow.
Graphene appears in composites that are stronger and lighter, in coatings that protect against corrosion, in sensors that detect minute changes, and in research laboratories exploring new physical phenomena. It may not have changed everything overnight, but it has changed how scientists think about materials and dimensions.
This quieter revolution may ultimately be more profound than the original hype. It reflects a deeper understanding of how progress unfolds, through accumulation rather than sudden transformation.
What Graphene Taught Us About Discovery
The story of graphene is, at its core, a lesson about discovery itself. It reminds us that nature still holds surprises, even in familiar elements like carbon. It shows that questioning assumptions can open entirely new fields of inquiry. It demonstrates that failure to meet inflated expectations does not diminish genuine scientific achievement.
Graphene also teaches humility. Even the most extraordinary material must contend with practical realities. Understanding those realities requires patience, collaboration, and persistence.
A Material That Changed How We Look at the World
Graphene may not have single-handedly transformed technology as once imagined, but it changed something deeper. It changed how we look at matter, how we imagine the limits of materials, and how we pursue innovation. It revealed that a single atomic layer could hold a universe of possibilities.
In the end, graphene’s greatest contribution may be the inspiration it provided. It reminded us that science is not just about answers but about questions. It showed that wonder still has a place in the laboratory, and that even the thinnest of materials can carry enormous weight in human understanding.
Graphene promised to change everything. In a quieter, more subtle way, it did.