By tapping into the brain’s fear circuits just moments after trauma, scientists may have found a fast-acting, female-specific way to stop PTSD before it starts.
In the complex aftermath of trauma, our brains have a tendency to hang onto fear—hard. This biological grip can sometimes tighten into posttraumatic stress disorder (PTSD), where even neutral cues—like a scent, a sound, or a casual phrase—become emotionally loaded triggers. But what if a single dose of a drug, administered within minutes after a traumatic experience, could soften the memory’s impact and prevent the condition from taking hold?
That’s precisely the question a team of neuroscientists from the Institut de Neurociències at the Universitat Autònoma de Barcelona set out to explore. And their answer, published in a new study in Brain Medicine, is compelling: one shot of the drug Osanetant, given shortly after a traumatic stressor, significantly reduced fear expression in female mice—paving the way for what could become a sex-specific, time-sensitive intervention for PTSD.
“We’re not preventing fear learning—but we’re reducing how intensely it gets biologically stored,”
– Neha Acharya and Jaime Fabregat, co-first authors
Interrupting the Fear Loop: How It Works
To understand how Osanetant works, you need to dive into the brain’s fear-processing circuits. Central to this process is the Tac2 pathway, a system involved in regulating emotional behavior. Osanetant targets a receptor in this pathway—the neurokinin 3 receptor (Nk3R)—effectively acting as a blocker or antagonist. By interfering with Nk3R activity, researchers aimed to disrupt how fear memories are encoded during the crucial early stages following trauma.
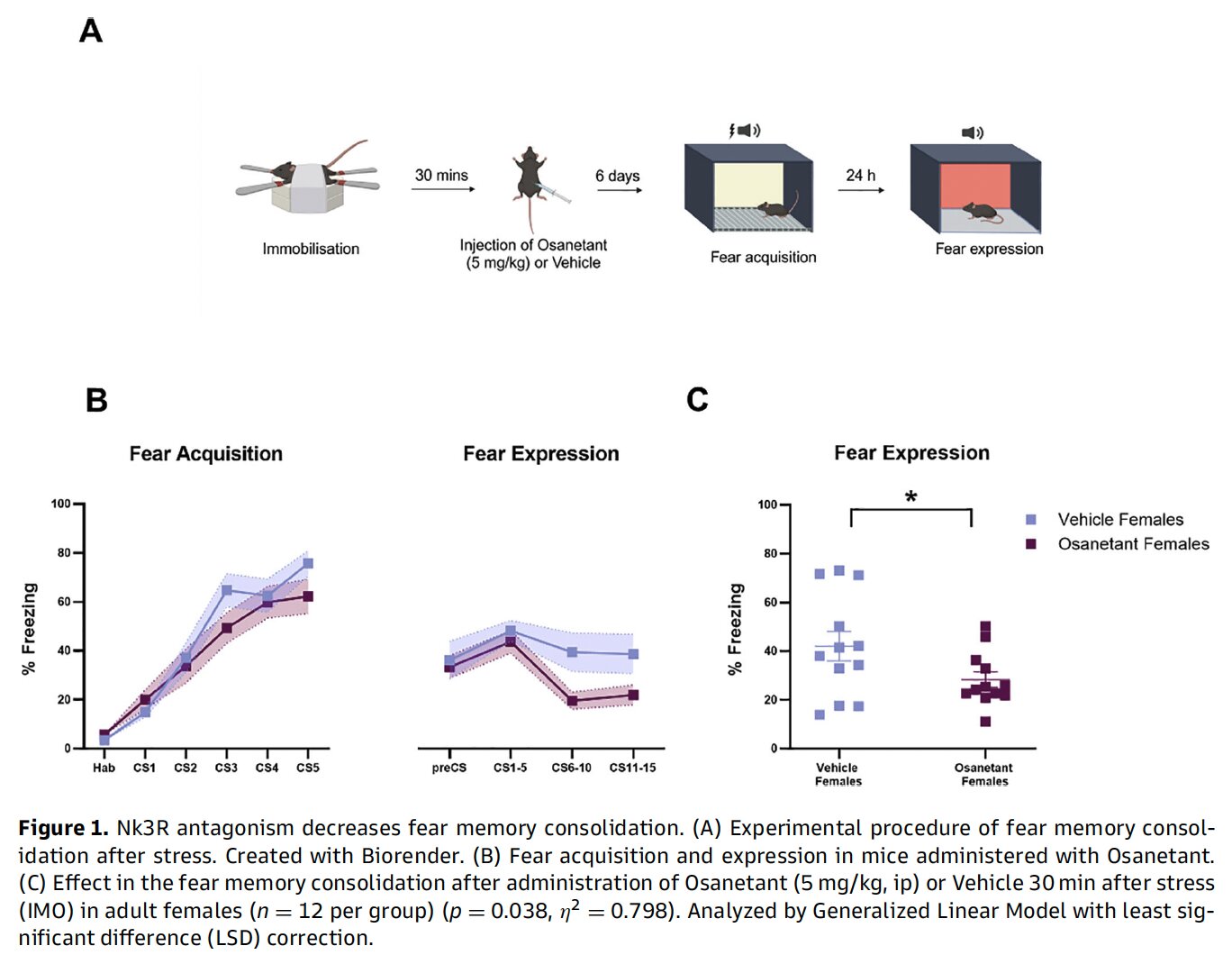
In the study, female mice were exposed to an intense immobilization stress—a validated model for PTSD-like conditions in rodents. Thirty minutes later, they received a single injection of Osanetant. Six days after that, the mice underwent classical fear-conditioning protocols.
The result? Mice that had been treated with Osanetant showed significantly less freezing behavior—a standard marker of fear—compared to untreated controls. In statistical terms, the difference was significant (p = 0.038), suggesting the drug had successfully weakened the consolidation of traumatic memory.
Why Female Mice? Tackling the Gender Gap in PTSD Research
One of the most striking features of this study is also one of its most long-overdue: its exclusive focus on female animals.
PTSD is nearly twice as common in women as in men. Yet, for decades, neuroscience has leaned heavily on male rodent models, often ignoring key sex differences in brain function and drug response. That means women have been largely left out of the experimental conversation—even when they’re the ones most at risk.
“We’ve known for years that female and male brains don’t process trauma the same way,”
– Dr. Raül Andero, senior author and principal investigator
Andero and his team aimed to correct that imbalance. By using only female mice, they were able to zero in on female-specific mechanisms—without male data obscuring the signal.
The implications are major. Female-focused pharmacological strategies remain rare, despite clear biological differences in how stress is processed and remembered. This study takes a meaningful first step toward designing PTSD interventions with sex as a central variable, not an afterthought.
The Role of Stress: When a Drug’s Effects Flip
Interestingly, Osanetant doesn’t always reduce fear. In fact, in earlier work from the same lab, the drug had the opposite effect in non-stressed female mice—increasing fear expression rather than reducing it.
So what changed?
The answer may lie in how trauma itself rewires the brain. After acute stress, neural circuits don’t just behave differently—they’re reprogrammed to respond in new ways. That could mean the same drug has drastically different outcomes depending on whether it’s delivered in a stressed or non-stressed state.
Several molecular suspects may be behind this shift: proteins like β-catenin, BDNF, GSK-3β, and mTOR are all known to influence synaptic plasticity and fear memory. While this study didn’t explore these pathways directly, future research may unravel how they interact with Nk3R activity—and why Osanetant becomes beneficial only after trauma tips the scales.
Could trauma ‘prime’ the brain to respond differently to drugs?
Does Nk3R antagonism only work when a certain stress threshold is crossed?
And crucially—can these effects be replicated in humans?
These are the questions that now animate the team’s future research.
A Time-Sensitive Tool with Real-World Potential
The biggest takeaway from the study may be the narrow, but powerful window during which Osanetant exerts its effect. Administered within 30 minutes after trauma, the drug seems to interfere with the biological consolidation of fear memories—essentially reducing the emotional “stickiness” of a traumatic event.
This kind of pharmacological precision is rare. Most current PTSD treatments—like SSRIs or trauma-focused therapy—come well after the fact, often when the disorder is already deeply entrenched.
But what if we could intervene before the fear takes hold?
Given that Osanetant has already been proven safe in clinical trials for other conditions, its repurposing for trauma prevention is a tantalizing prospect. The researchers envision a scenario where the drug could be administered in emergency rooms—after a car accident, sexual assault, or combat exposure—to act as a pharmacological shield against over-consolidated trauma memories.
“This is an especially important window,”
– Acharya and Fabregat emphasize
By dampening the emotional intensity of traumatic memories early on, Osanetant might not erase the memory—but it could reduce the chance that it morphs into full-blown PTSD.
Limitations and Next Steps
Of course, no study is perfect. The researchers acknowledge several limitations: the study only used female mice, didn’t track estrous cycles (which can influence hormonal states), and didn’t examine molecular markers or brain changes.
But the behavioral outcome—a clear, statistically significant reduction in fear expression—is robust. And that’s enough to justify larger studies with more diverse models, molecular analysis, and eventually, clinical trials in humans.
The next frontier? Testing whether Osanetant works in real-life trauma scenarios. If the drug’s timing is critical, getting it into emergency protocols could be challenging—but not impossible. With PTSD affecting millions worldwide, the incentive to find fast, effective interventions is huge.
Final Thoughts: A New Era in PTSD Prevention?
We’re just beginning to understand how trauma reshapes the brain—and how to stop it from leaving lasting scars. But this study offers a rare glimpse into what true prevention might look like: a targeted drug, delivered in the right moment, in the right brain.
And for women—who remain underrepresented in both research and treatment—the findings feel especially urgent.
Science still has a long road ahead. But if Osanetant holds up in future studies, we may soon enter an era where PTSD isn’t just managed, but stopped before it ever begins.
Reference: NK3R antagonism reduces fear expression in a PTSD-like model of female mice, Brain Medicine (2025). DOI: 10.61373/bm025l.0035