For decades, scientists have known that our DNA holds far more than just the instructions for building proteins. In fact, only about 1–2% of the human genome encodes genes in the traditional sense. The remaining 98%—long labeled as “junk DNA”—is anything but junk. This vast and mysterious territory, sometimes called the “dark genome,” is now emerging as a powerful regulator of health and disease.
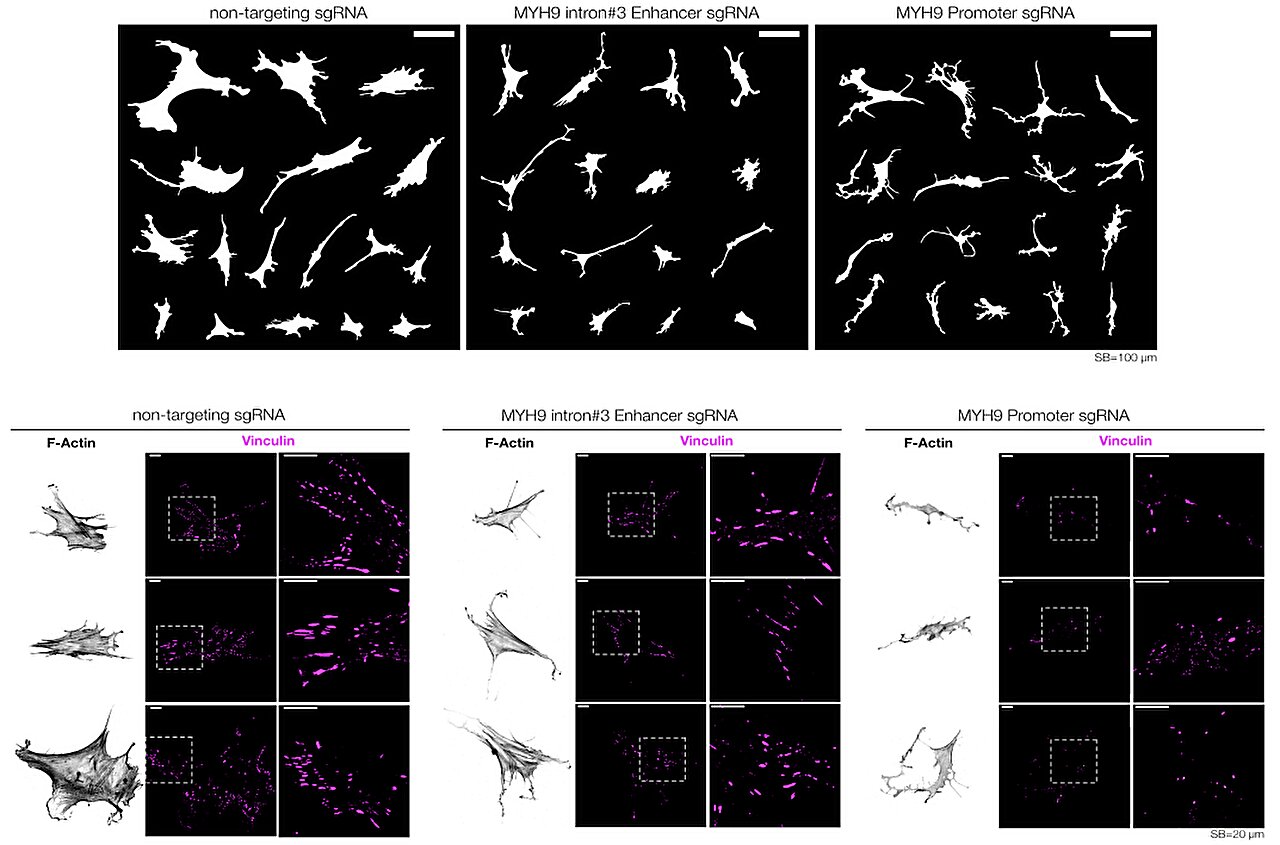
Researchers at Duke University have illuminated a new part of this hidden landscape. Using state-of-the-art CRISPR technologies, they have identified stretches of DNA that control how cells sense and respond to the physical properties of their environment. These DNA elements, which they call mechanoenhancers, appear to act as switches that help cells interpret mechanical signals, such as stiffness or pressure, and translate them into biological outcomes.
Their findings, published in Science, could reshape our understanding of how diseases like cancer, fibrosis, stroke, neurodegeneration, and even aging arise—and, importantly, how they might one day be treated.
The Forgotten Language of Mechanics
For much of modern biology, chemical signals have dominated the conversation. Scientists have developed deep insights into how hormones, cytokines, and pharmaceutical drugs can alter gene expression and direct cellular function. But a cell’s physical environment—how rigid or soft the surrounding tissue is, what mechanical forces are pressing upon it—has often remained in the shadows.
Yet these physical cues are every bit as influential as chemical ones. Tissue stiffness, for example, changes dramatically in fibrosis, where organs scar and harden, or in cancer, where tumors create altered mechanical landscapes. As Charlie Gersbach, Director of the Duke Center for Advanced Genomic Technologies, explains, “Mechanical stimuli from a cell’s microenvironment are potent regulators of growth, death, differentiation, and migration.”
In other words, the way a cell “feels” its environment can shape its destiny. Until recently, though, the genetic mechanisms linking physical forces to cellular behavior were almost impossible to untangle.
CRISPR as a Genomic Lantern
That began to change with the rise of CRISPR—a revolutionary tool for editing DNA. By cutting and disabling specific DNA sequences, scientists can test whether those sequences are important and how they influence cellular behavior.
At Duke, Gersbach and colleague Gregory Crawford joined forces with mechanobiology expert Brent Hoffman to probe how the dark genome might regulate responses to mechanical forces. Their team cultured cells on hydrogels designed to mimic tissues of varying stiffness, essentially creating artificial environments where cells could “feel” different physical conditions.
Within just 20 hours, the cells showed dramatic changes: thousands of genes altered their expression, and nearly 50,000 regions of the genome changed their structure. This rapid and sweeping response underscored how profoundly the mechanical environment shapes cellular biology.
But which of these changes truly mattered? That’s where CRISPR came in. By systematically silencing different regions of the dark genome, the researchers were able to identify enhancers—DNA switches—that directly affected cell growth, migration, and gene regulation under different mechanical conditions.
Discovering Mechanoenhancers
The team’s breakthrough came when they found a subset of enhancers that responded specifically to mechanical cues. These mechanoenhancers turned out to be critical in controlling how cells adjusted to the stiffness of their surroundings. When these regions were silenced, cells lost their ability to properly adapt, with ripple effects on gene activity and cellular behavior.
To dig deeper, the researchers collaborated with other experts at Duke. Yarui Diao used cutting-edge techniques to show that mechanoenhancers physically change how they interact with their target genes depending on the cell’s environment. Meanwhile, Purushothama Tata contributed samples from lung biopsies of patients with idiopathic pulmonary fibrosis (IPF). Strikingly, the mechanoenhancers identified in the lab were also active in diseased human tissues, directly linking them to pathological changes.
This connection suggests that mechanoenhancers could be key players in conditions where tissue mechanics go awry—whether in fibrotic lungs, stiffened arteries, or solid tumors.
Why It Matters for Human Health
The implications of this research are vast. If mechanoenhancers help cells interpret mechanical signals, then they represent potential new drug targets. Treatments could one day be designed to dial down harmful responses in stiffened, fibrotic tissues or prevent tumors from exploiting mechanical cues to fuel their growth.
Even more intriguingly, mechanoenhancers might help explain some of the mysteries of aging and neurodegeneration. As tissues change their mechanical properties over time, these DNA switches may alter how cells behave in ways that contribute to decline or disease. By mapping and eventually manipulating these sequences, scientists may be able to slow or even reverse such processes.
As Brian Cosgrove, the study’s lead postdoctoral fellow, put it, “Mapping these mechanoenhancers can improve our mechanistic understanding of diseases that involve changes to tissue mechanical properties, like fibrosis and cancer, and possibly lead to new drug targets or methods for engineering how cells sense pathologic mechanical environments.”
The Next Frontier
The Duke team’s work is only the beginning. Ongoing projects aim to explore how mechanoenhancers vary across different cell types, disease conditions, and stages of life. They are also investigating how natural genetic variation in these DNA sequences might make some individuals more resilient to mechanical stresses, while others are more vulnerable.
Perhaps most exciting, researchers envision directly targeting mechanoenhancers with genetic medicines—tools that can precisely switch them off or modulate their activity. Such therapies could offer new hope for patients with conditions that have long resisted treatment.
Gersbach emphasizes that this research represents not only a scientific breakthrough but also a model for collaboration: “This project highlights what can happen when engineering, medicine, and biology intersect. By combining innovative technologies with patient-derived samples, we are uncovering fundamental mechanisms with real potential to impact human health.”
Reimagining the Genome
The discovery of mechanoenhancers underscores a broader lesson: the genome is not just a static instruction manual, but a dynamic, responsive system deeply intertwined with the environment. The so-called dark genome is, in reality, a rich and complex regulatory network that we are only beginning to understand.
With every advance in tools like CRISPR, epigenetic profiling, and mechanobiology, we illuminate more of this once-hidden territory. And with each new discovery, we come closer to reimagining human health and disease not as fixed outcomes, but as processes that can be understood, influenced, and even rewritten.
The genome, it turns out, is not silent. It is listening—listening to chemistry, listening to physics, and, as Duke’s work now shows, listening to the very mechanics of life itself.
More information: Brian D. Cosgrove et al, Mechanosensitive genomic enhancers potentiate the cellular response to matrix stiffness, Science (2025). DOI: 10.1126/science.adl1988