Every night, whether we want to or not, sleep takes us. It folds us into darkness, dulls our senses, and pulls the curtain on our conscious lives. For decades, science has known that sleep is essential—but not why. We know it restores us, consolidates memory, and clears cellular debris in the brain. But none of that has ever fully explained what drives the need to sleep. What, exactly, flips the internal switch from alertness to slumber?
Now, a team of researchers from the University of Oxford has illuminated that mystery—by looking deep into the powerhouses of brain cells. Their new study, published in Nature, doesn’t just offer a theory about sleep—it proposes a physical trigger, rooted in the very electricity that keeps us alive.
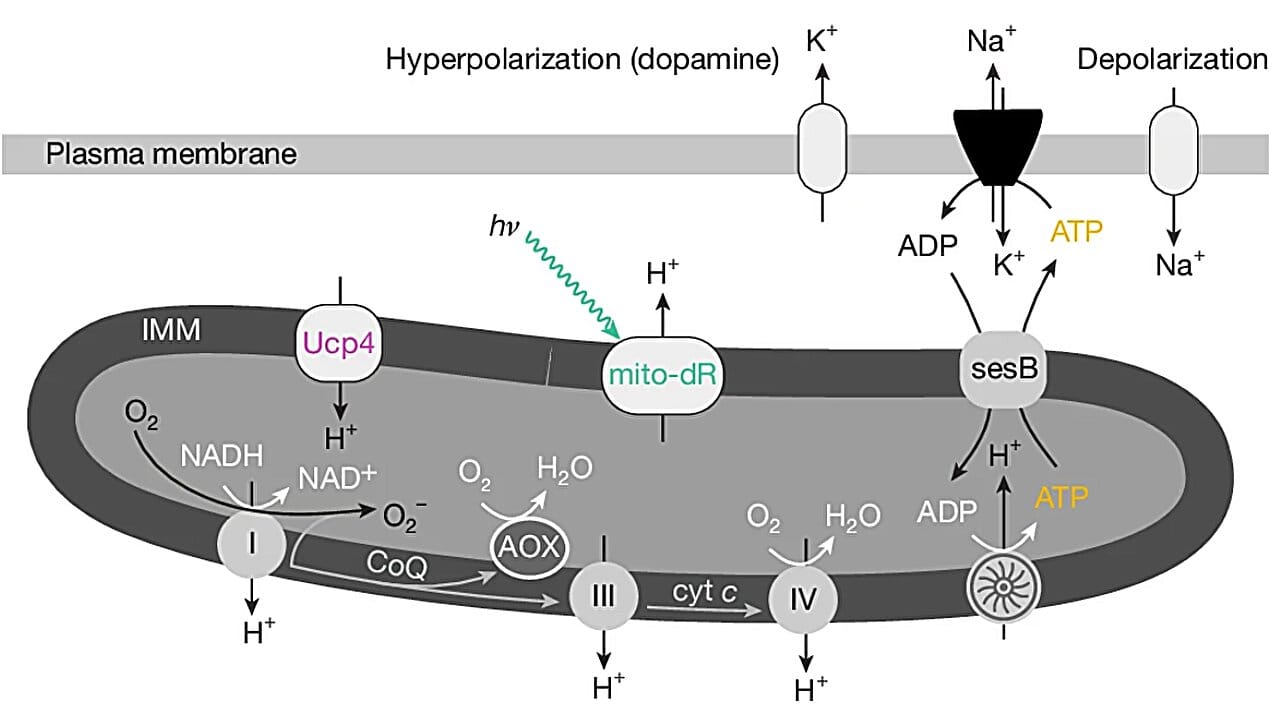
The answer, it turns out, lies in mitochondria, the microscopic generators that fuel every action we take, from blinking to breathing. When these generators in certain brain cells overheat, they leak electrons. And when enough of those electrons escape, the brain shuts down—not out of laziness, but out of survival. Sleep, it seems, may be the body’s built-in emergency brake, preventing the brain’s power grid from melting down.
Where Electricity Meets Exhaustion
Professor Gero Miesenböck and Dr. Raffaele Sarnataro led the Oxford team behind this revelation, focusing their work on Drosophila melanogaster—the humble fruit fly. Despite their tiny size, fruit flies share surprisingly similar genetic and cellular architecture with humans, especially in the brain. This makes them ideal models for understanding basic biological processes.
The researchers targeted specialized neurons known to regulate sleep. These cells, found deep in the fly brain, act like sensors. They don’t just monitor the world around the organism—they monitor the internal world too. What they discovered was extraordinary.
Inside these neurons, mitochondria convert oxygen and nutrients into energy through a process called aerobic respiration. It’s a vital function, like charging a battery. But when the cells receive more fuel than they need—too much oxygen, too many nutrients—the mitochondria start to leak electrons.
This leak produces reactive oxygen species (ROS), unstable molecules that can damage proteins, DNA, and cell membranes. The damage isn’t immediate or explosive—it’s more like a slow corrosion. But left unchecked, it can spiral into catastrophe for brain cells.
So, the brain steps in.
Those specialized neurons detect the electron leak, recognize it as a warning, and trip the circuit. The result is a cascade of neurological events that lead to sleep. Not to dream. Not to rest. But to halt the damage—to cool down the system before it burns out.
Sleep as a Safety Shutdown
Imagine a modern power station. As it produces electricity, a certain amount of heat and waste are inevitable. But if the system is overloaded—too much current, too fast—the generators begin to overheat, threatening to destroy the entire grid. That’s when emergency systems kick in, shutting down parts of the plant to prevent disaster.
Now zoom back into the brain. The same principle is at work. When energy production in these sleep-related neurons hits dangerous levels, the electron leak becomes a red flag. It’s not fatigue that triggers sleep—it’s a kind of metabolic emergency protocol. The brain is protecting itself from self-destruction.
This subtle but powerful mechanism reframes how we think about sleep. No longer is it just a passive state of rest—it’s an active response to internal electrical stress. Without it, the energy demands of our waking lives could leave lasting scars on the very systems we rely on to think, move, and feel.
Manipulating Sleep at the Molecular Level
To test their theory, the Oxford team didn’t stop at observation. Using genetic tools and optogenetics—a technique that uses light to control cells—they manipulated the mitochondria within these neurons. By increasing or decreasing the flow of electrons, they were able to directly control how much the flies slept.
When they increased energy supply artificially, mitochondria leaked more electrons, and the flies became sleepier. When they reduced mitochondrial output, the flies stayed awake longer. The researchers even replaced the brain’s natural energy source with light-activated proteins borrowed from microorganisms. The result was the same: more energy caused more electron leakage, which in turn triggered more sleep.
This level of control isn’t just a scientific achievement—it’s a window into how sleep might be managed in humans, especially in disorders where sleep is disrupted or absent. If the pressure to sleep is linked to energy imbalance, could we someday correct that imbalance with medicine—or even with targeted light therapy?
Why It Matters Beyond Sleep
The implications of this discovery ripple far beyond bedtime.
There has long been a mysterious link between metabolism, lifespan, and sleep. Smaller animals like mice and hummingbirds have sky-high metabolic rates. They burn through oxygen and calories quickly, sleep more often, and live shorter lives. Larger animals, with slower metabolisms, tend to sleep less and live longer. Until now, that relationship was poorly understood.
This study provides a potential explanation. High metabolism means more mitochondrial activity, more electron leakage, and therefore more frequent triggering of sleep. Over a lifetime, that high-energy lifestyle may also mean more accumulated cellular damage, leading to faster aging.
It may also shed light on mitochondrial diseases—a range of conditions caused by defects in energy production. Patients with these disorders often suffer chronic fatigue, not because their muscles are weak, but because their brain cells may be constantly signaling for rest. If the sleep mechanism is tied to electron leakage, these individuals might be caught in a perpetual energy crisis—unable to stay “online” for long without overwhelming their system.
Rethinking What Sleep Is For
For generations, scientists asked why sleep feels so necessary. We now have an answer that goes deeper than any psychological explanation. According to this research, sleep is not a choice—it’s a consequence. A reaction to a buildup of internal strain. A daily reset button pressed by cells that sense they’re reaching a critical threshold.
Professor Miesenböck put it best: “We set out to understand what sleep is for, and why we feel the need to sleep at all. Despite decades of research, no one had identified a clear physical trigger. Our findings show that the answer may lie in the very process that fuels our bodies: aerobic metabolism.”
Sleep, in this light, is not a luxury. It’s not a passive gap in productivity. It’s essential upkeep for a brain that runs hot and fast. It’s the moment when our neural generators power down, clear out the waste, cool the circuits, and prepare to spark again.
Into the Future of Sleep Science
This breakthrough reorients the field of sleep science in a profound way. Instead of asking what sleep does for us, we can now ask what happens to us without it—on a molecular level. And that opens the door to new therapies for insomnia, narcolepsy, chronic fatigue, and even neurodegenerative diseases like Alzheimer’s, where mitochondrial dysfunction is a key feature.
It also challenges how society views sleep deprivation. Skipping rest isn’t a badge of honor—it’s a gamble with your brain’s circuitry. Without sleep, we’re not just tired. We’re teetering on the edge of an electrical meltdown.
There’s poetry in the idea that one of the most mysterious aspects of our biology—sleep—is actually governed by a kind of microscopic crisis. That every night, as our minds surrender to dreams, our cells are performing a delicate dance of energy management and self-preservation.
Sleep is not the opposite of power. It is its guardian.
Reference: Raffaele Sarnataro et al, Mitochondrial origins of the pressure to sleep, Nature (2025). DOI: 10.1038/s41586-025-09261-y